| CPC A61N 1/39622 (2017.08) [A61N 1/0587 (2013.01); A61N 1/3622 (2013.01); A61N 1/3756 (2013.01)] | 22 Claims |
|
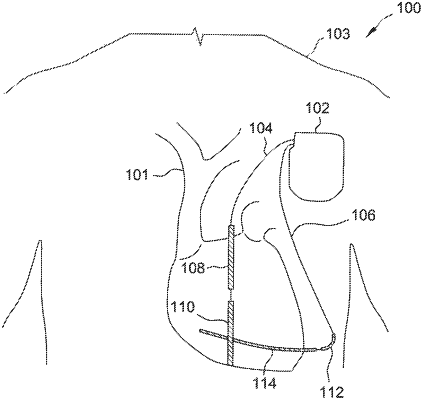
1. A subcutaneous implantable cardioverter-defibrillator (SICD) comprising:
one or more leads configured to be implanted in a subcutaneous region of a subject, the one or more leads including multiple electrodes;
a case forming one of the multiple electrodes;
sensing circuitry; and
a controller configured to:
apply anti-tachycardia pacing (ATP) therapy to the subject via a pacing combination of electrodes at a first time, the pacing combination of electrodes comprising two or more of the multiple electrodes;
connect the sensing circuitry to a first sensing combination of electrodes, the first sensing combination of electrodes comprising two or more of the multiple electrodes;
sense, via the first sensing combination of electrodes, a first paced evoked response that is responsive to the ATP therapy applied at the first time;
apply anti-tachycardia pacing (ATP) therapy to the subject via a same or different pacing combination of electrodes at a second time;
connect the sensing circuitry to a second sensing combination of electrodes, the second sensing combination of electrodes comprising two or more of the multiple electrodes;
sense, via the second sensing combination of electrodes, a second paced evoked response that is responsive to the ATP therapy applied at the second time;
determine sizes of the first and second paced evoked responses; and
select one of the first sensing combination of electrodes or the second sensing combination of electrodes to use for subsequent evoked response detection based on the sizes of the first and second paced evoked responses.
|