| CPC A61L 26/0038 (2013.01) [A61L 26/0004 (2013.01); A61L 26/0023 (2013.01); A61L 26/0047 (2013.01); A61L 2400/04 (2013.01)] | 5 Claims |
|
1. A liquid medical material comprising:
a gelatin aqueous solution including calcium at a concentration of 0.2 M or more and 1.0 M or less, and gelatin having a concentration of 5% by weight or more and 40% by weight or less, an average molecular weight of 80,000 or more and 120,000 or less, and a molecular weight distribution of 20,000 or more and 300,000 or less;
transglutaminase configured to induce crosslinking of the gelatin; and
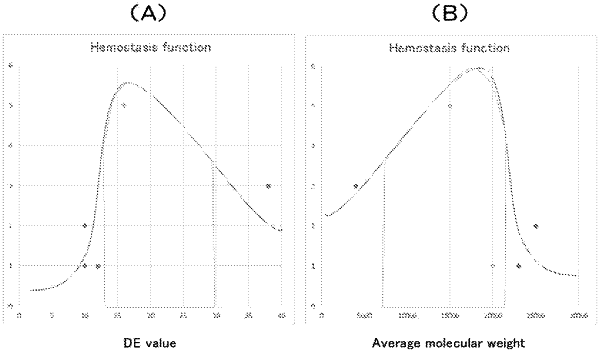
porous dextran having a DE value of 10 or more and 25 or less, and an average molecular weight of 5000 or more and 25000 or less,
wherein the calcium includes a compound selected from the group consisting of calcium chloride and calcium carbonate wherein the liquid material is hemostatic material.
|