| CPC A61K 9/4858 (2013.01) [A61K 9/4808 (2013.01); A61K 9/4825 (2013.01); A61K 9/4833 (2013.01); A61K 31/4412 (2013.01)] | 18 Claims |
|
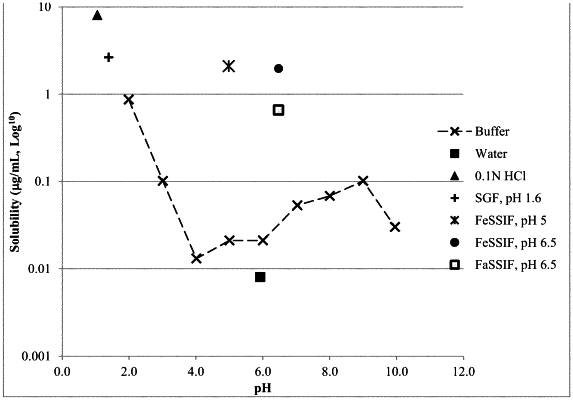
1. A solid solution capsule formulation comprising Compound 1 as a free base, in its neutral form, or in the form of a pharmaceutically acceptable salt
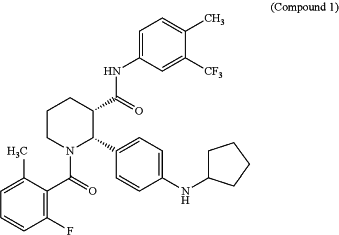 and a vehicle comprising
at least one non-ionic surfactant selected from the group consisting of macrogol-40-glycerol hydroxystearate, macrogolglycerol ricinoleate, and macrogol-15-hydroxystearate, and
at least one water-soluble solubilizer selected from the group consisting of PEG-1500, PEG-1540, PEG-2000, PEG-3000, PEG-3350, PEG-4000, PEG-6000, PEG-8000,
wherein
Compound 1 comprises about 1 to 3% by weight of the total fill weight of said solid solution capsule formulation, the vehicle comprises about 97 to 99% by weight of the total fill weight of said solid solution capsule formulation, and
the total weight of the vehicle comprises a 45:55 to 55:45 ratio of the at least one non-ionic surfactant to the at least one water-soluble solubilizer.
|