| CPC A61K 31/675 (2013.01) [A61K 9/0019 (2013.01); A61K 9/0048 (2013.01); A61P 27/02 (2018.01)] | 33 Claims |
|
1. A method of treating a patient suffering from a retinal vasculopathy comprising administering to the patient a therapeutically effective dose of a meglumine salt of a compound of Formula I (Compound-meglumine):
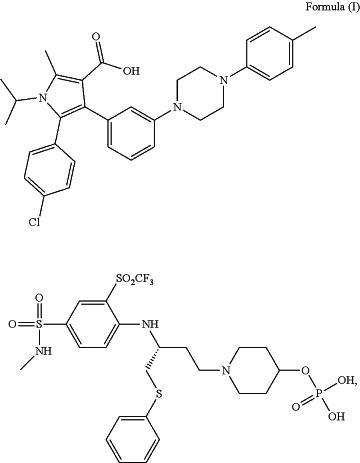 (R)-5-(4-chlorophenyl)-1-isopropyl-2-methyl-4-(3-(4-(4-((4-((1-(phenylthio)-4-(4-((phosphonooxy)methyl)piperidin-1-yl)butan-2-yl)amino)-3-((trifluoromethyl)sulfonyl)phenyl)sulfonamido)phenyl)piperazin-1-yl)phenyl)-1H-pyrrole-3-carboxylic acid,
wherein the therapeutically effective dose is up to 10 ug of the Compound-meglumine is administered intravitreally (IVT) per eye.
|