| CPC A61K 31/5377 (2013.01) [A61K 31/4412 (2013.01); A61K 31/444 (2013.01); A61K 31/475 (2013.01); A61K 31/573 (2013.01); A61K 31/675 (2013.01); A61K 31/704 (2013.01); A61K 39/39558 (2013.01); A61K 45/06 (2013.01); A61P 35/00 (2018.01); G01N 33/57407 (2013.01)] | 14 Claims |
|
1. A method for treating a germinal center-derived lymphoma in a subject in need thereof comprising administering a therapeutically effective amount of an EZH2 inhibitor and a therapeutically effective amount of a standard of care agent, wherein the EZH2 inhibitor is of Formula (I):
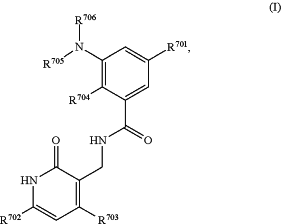 or a pharmaceutically acceptable salt thereof; wherein:
R701 is H, F, OR707, NHR707, —(C≡C)—(CH2)n7—R708, phenyl, 5- or 6-membered heteroaryl, C3-8 cycloalkyl, or 4-7 membered heterocycloalkyl containing 1-3 heteroatoms, wherein the phenyl, 5- or 6-membered heteroaryl, C3-8 cycloalkyl or 4-7 membered heterocycloalkyl each independently is optionally substituted with one or more groups selected from halo, C1-3 alkyl, OH, O—C1-6 alkyl, NH—C1-6 alkyl, and, C1-3 alkyl substituted with C3-8 cycloalkyl or 4-7 membered heterocycloalkyl containing 1-3 heteroatoms, wherein each of the O—C1-6 alkyl and NH—C1-6 alkyl is optionally substituted with hydroxyl, O—C1-3 alkyl or NH—C1-3 alkyl, each of the O—C1-3 alkyl and NH—C1-3 alkyl being optionally further substituted with O—C1-3 alkyl or NH—C1-3 alkyl;
each of R702 and R703 independently is H, halo, C1-4 alkyl, C1-6 alkoxyl or C6-C10 aryloxy, each optionally substituted with one or more halo;
each of R704 and R705 independently is C1-4 alkyl;
R706 is cyclohexyl substituted by N(C1-4 alkyl)2 wherein one or both of the C1-4 alkyl is optionally substituted with C1-6 alkoxy; or R706 is tetrahydropyranyl;
R707 is C1-4 alkyl optionally substituted with one or more groups selected from hydroxyl, C1-4 alkoxy, amino, mono- or di-C1-4 alkylamino, C3-8 cycloalkyl, and 4-7 membered heterocycloalkyl containing 1-3 heteroatoms, wherein the C3-8 cycloalkyl or 4-7 membered heterocycloalkyl each independently is further optionally substituted with C1-3 alkyl;
R708 is C1-4 alkyl optionally substituted with one or more groups selected from OH, halo, and C1-4 alkoxy, 4-7 membered heterocycloalkyl containing 1-3 heteroatoms, or O—C1-6 alkyl, wherein the 4-7 membered heterocycloalkyl can be optionally further substituted with OH or C1-6 alkyl; and
n7 is 0, 1, or 2; and
wherein the standard of care agent is selected from Alisertib, Enzastaurin, Vemurafenib, Ruxolitinib, Fedratinib, Tofacitinib, OG-L002, and GSK J4.
|