| CPC G16H 20/17 (2018.01) [G16H 20/13 (2018.01); G16H 40/67 (2018.01); H04W 4/029 (2018.02)] | 24 Claims |
|
1. A removable tracking device, comprising:
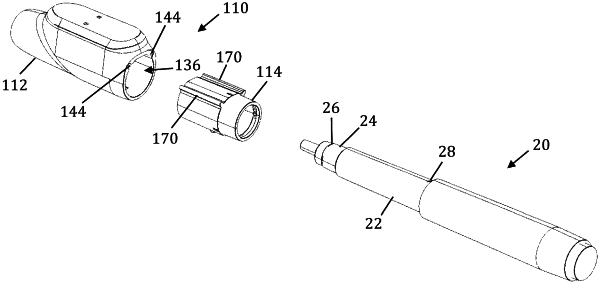
a cap configured to releasably couple to a distal end portion of a medicine dispensing device, the cap defining an interior cavity between an open proximal end configured to receive the distal end portion of the medicine dispensing device and a closed distal end configured to protect a needle of the medicine dispensing device when the cap is coupled to the distal end portion of the medicine dispensing device;
an interchangeable coupler adapter configured to enable coupling of the cap to a plurality of medicine dispensing devices of different geometries, the coupler adapter having a first end portion configured to be received within the interior cavity defined by the cap for removably coupling the coupler adapter to the cap and a second end portion defining an axial lumen configured to accommodate the distal end portion of at least one medicine dispensing device of the plurality of medicine dispensing devices of different geometries for removably coupling the distal end portion of the at least one medicine dispensing device to the coupler adapter and the cap;
a detection switch disposed within the cap and configured to detect:
removal of the cap from the medicine dispensing device in response to the distal end portion of the medicine dispensing device being removed from the interior cavity of the cap; and
coupling of the cap to the medicine dispensing device in response to the distal end portion of the medicine dispensing device being received within the interior cavity of the cap;
a processor disposed within the cap and configured to process at least one of time data or dose data corresponding to a dose of medicine dispensed from the medicine dispensing device; and
a storage module disposed within the cap and storing instructions which, when executed by the processor, cause performance of:
determining whether an activation event occurred within a predetermined period of time that includes comparing the time of the activation event to the predetermined period of time, and upon a determination that the dose of medicine was dispensed within the predetermined period of time, identifying the dispensing of the dose of medicine as a safe dosing condition, and upon a determination that the activation event occurred outside of the predetermined period of time, identifying the activation event as an unsafe dosing condition; and
displaying, on a user interface of the cap, a first visual indication to indicate an identified safe dosing condition and a second visual indication to indicate an identified unsafe dosing condition, the second visual indication being different than the first visual indication.
|