| CPC C07K 14/005 (2013.01) [A61K 9/51 (2013.01); A61K 39/145 (2013.01); C12N 7/00 (2013.01); C07K 2319/02 (2013.01); C12N 2760/16122 (2013.01); C12N 2760/16123 (2013.01); C12N 2760/16134 (2013.01); C12N 2760/16222 (2013.01); C12N 2760/16223 (2013.01); C12N 2760/16234 (2013.01)] | 12 Claims |
|
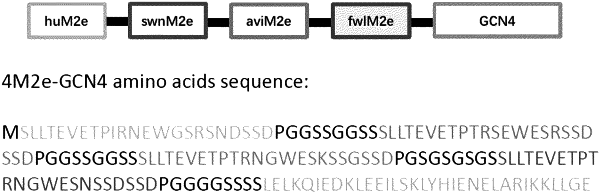
1. A nanoparticle formed by crosslinking a fusion protein with a crosslinking agent and/or desolvating the fusion protein with a desolvating agent, wherein the fusion protein comprises a series of 2 to 8 influenza virus matrix protein 2 extracellular (M2e) domains, wherein the series of 2 to 8 influenza virus M2e domains is linked to a multimerization domain, wherein the fusion protein further comprises influenza neuraminidase (NA) protein linked to the multimerization domain.
|