| CPC C07D 413/14 (2013.01) [A61K 31/506 (2013.01); A61P 35/00 (2018.01); C07D 409/14 (2013.01); C07D 417/14 (2013.01); C07D 471/04 (2013.01); C07D 471/10 (2013.01); C07D 487/04 (2013.01); C07D 487/08 (2013.01); C07D 487/10 (2013.01)] | 39 Claims |
|
1. A compound of the formula (I)
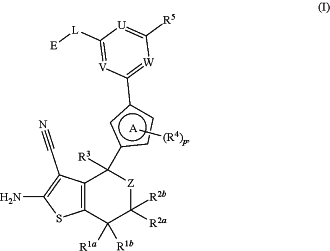 wherein
R1a and R1b are both independently selected from the group consisting of hydrogen, C1-4alkyl, C1-4haloalkyl, C1-4alkoxy, C1-4haloalkoxy, halogen, —NH2, —NH(C1-4alkyl), —N(C1-4alkyl)2, C3-5cycloalkyl and 3-5 membered heterocyclyl;
R2a and R2b are both independently selected from the group consisting of hydrogen, C1-4alkyl, C1-4haloalkyl, C1-4alkoxy, C1-4haloalkoxy, halogen, —NH2, —NH(C1-4alkyl), —N(C1-4alkyl)2, C3-5cycloalkyl and 3-5 membered heterocyclyl;
and/or, optionally, one of R1a or R1b and one of R2a or R2b together with the carbon atoms they are attached form a cyclopropane ring;
Z is —(CR6aR6b)n—;
each R6a and R6b is independently selected from the group consisting of hydrogen, C1-4alkyl, C1-4haloalkyl, C1-4alkoxy, C1-4haloalkoxy, halogen, —NH2, —NH(C1-4alkyl), —N(C1-4alkyl)2, C3-5cycloalkyl and 3-5 membered heterocyclyl;
n is selected from the group consisting 0, 1 and 2;
R3 is selected from the group consisting of hydrogen, C1-6alkyl, C1-6haloalkyl, C1-6alkoxy, C1-6haloalkoxy, cyano-C1-6alkyl, halogen, —OH, —NH2, —NH(C1-4alkyl), —N(C1-4alkyl)2, —CN, C3-5cycloalkyl and 3-5 membered heterocyclyl;
ring A is a ring selected from the group consisting of pyrrole, furan, thiophene, imidazole, pyrazole, oxazole, isoxazole, thiazole, isothiazole and triazole;
each R4, if present, is independently selected from the group consisting of C1-6alkyl, C1-6haloalkyl, C1-6alkoxy, C1-6haloalkoxy, cyano-C1-6alkyl, halogen, —OH, —NH2, —NH(C1-4alkyl), —N(C1-4alkyl)2, —CN, C3-5cycloalkyl and 3-5 membered heterocyclyl;
p is selected from the group consisting 0, 1, 2 and 3;
U is selected from the group consisting of nitrogen (═N—) and carbon substituted with RA (═C(RA)—);
V is selected from the group consisting of nitrogen (═N—) and carbon substituted with RB (═C(RB)—);
W is selected from the group consisting of nitrogen (═N—) and carbon substituted with Rc (═C(RC)—);
RA, RB and RC is each independently selected from the group consisting of hydrogen, to C1-6haloalkyl, C2-6alkynyl optionally substituted with C3-5cycloalkyl, C1-6alkoxy, C1-6haloalkoxy, halogen, —CN, —OH, —NH2, —NH(C1-4alkyl), —N(C1-4alkyl)2, —C(═O)NH2, —C(═O)NH(C1-4alkyl), —C(═O)N(C1-4alkyl)2, —S—C1-6alkyl, —S(═O)2—C1-6alkyl, C3-5cycloalkyl, 3-5 membered heterocyclyl and C1-6alkyl optionally substituted with a substituent selected from the group consisting of C1-6alkoxy, —CN, —OH, —NH2, —NH(C1-4alkyl), —N(C1-4alkyl)2, —C(═O)NH2, —C(═O)NH(C1-4alkyl) and —C(═O)N(C1-4alkyl)2;
R5 is selected from the group consisting of Ra1 and Rb1;
Ra1 is selected from the group consisting of C1-6alkyl, C1-6haloalkyl, C2-6alkenyl, C2-6alkynyl, C3-10cycloalkyl, C4-10cycloalkenyl, 3-11 membered heterocyclyl, C6-10aryl and 5-10 membered heteroaryl, wherein the C1-6alkyl, C1-6haloalkyl, C2-6alkenyl, C2-6alkynyl, C3-10cycloalkyl, C4-10cycloalkenyl, 3-11 membered heterocyclyl, C6-10aryl and 5-10 membered heteroaryl are all optionally substituted with one or more, identical or different Rb1 and/or Rc1;
each Rb1 is independently selected from the group consisting of —ORc1, —NRc1Rc1, halogen, —CN, —C(═O)Rc1, —C(═O)ORc1, —C(═O)NRc1Rc1, —S(═O)2Rc1, —S(═O)2NRc1Rc1, —NHC(═O)Rc1, —N(C1-4alkyl)C(═O)Rc1, —NHS(═O)2Rc1, —N(C1-4alkyl)S(═O)2Rc1, —NHC(═O)ORc1, —N(C1-4alkyl)C(═O)ORc1 and the bivalent substituent ═O;
each Rc1 is independently selected from the group consisting of hydrogen, C1-6alkyl, C1-6haloalkyl, C2-6alkenyl, C2-6alkynyl, C3-10cycloalkyl, C4-10cycloalkenyl, 3-11 membered heterocyclyl, C6-10aryl and 5-10 membered heteroaryl, wherein the C1-6alkyl, C1-6haloalkyl, C2-6alkenyl, C2-6alkynyl, C3-10cycloalkyl, C4-10cycloalkenyl, 3-11 membered heterocyclyl, C6-10aryl and 5-10 membered heteroaryl are all optionally substituted with one or more, identical or different Rd1 and/or Re1;
each Rd1 is independently selected from the group consisting of —ORe1, —NRe1Re1, halogen, —CN, —C(═O)Re1, —C(═O)ORe1, —C(═O)NRe1Re1, —S(═O)2Re1, —S(═O)2NRe1Re1, —NHC(═O)Re1, —N(C1-4alkyl)C(═O)Re1, —NHS(═O)2Rc1, —N(C1-4alkyl)S(═O)2Rc1, —NHC(═O)ORe1, —N(C1-4alkyl)C(═O)ORe1 and the bivalent substituent ═O;
each Re1 is independently selected from the group consisting of hydrogen, C1-6alkyl, C1-6haloalkyl, C2-6alkenyl, C2-6alkynyl, C3-10cycloalkyl, C4-10cycloalkenyl, 3-11 membered heterocyclyl, C6-10aryl and 5-10 membered heteroaryl, wherein the C1-6alkyl, C1-6haloalkyl, C2-6alkenyl, C2-6alkynyl, C3-10cycloalkyl, C4-10cycloalkenyl, 3-11 membered heterocyclyl, C6-10aryl and 5-10 membered heteroaryl are all optionally substituted with one or more, identical or different substituent(s) selected from the group consisting of C1-6alkyl, C1-6haloalkyl, C3-10cycloalkyl, 3-11 membered heterocyclyl optionally substituted with one or more, identical or different C1-4alkyl, C6-10aryl, 5-10 membered heteroaryl, —OH, C1-6alkoxy, C1-4alkoxy-C1-4alkyl, hydroxy-C1-4alkyl, halogen, —CN, —NH2, —C(═O)C1-4alkyl, —NH(C1-4alkyl), —N(C1-4alkyl)2 and the bivalent substituent ═O;
L is -L1-L2-L3-, wherein L1 is linked to E;
L1 is selected from the group consisting of a bond, —NH—, —N(C1-4alkyl)-, —O—, —C(═O)—, —NH—C(═O)—, —N(C1-4alkyl)-C(═O)—, —C(═O)—NH—, —C(═O)—N(C1-4alkyl)-, —C(═O)—, C1-6alkylen, C3-7cycloalkylene, phenylene, 4-12 membered heterocyclylene and 5-10 membered heteroarylene;
L2 is selected from the group consisting of C1-6alkylen, C3-7cycloalkylene, phenylene, 4-12 membered heterocyclylene and 5-10 membered heteroarylene;
L3 is selected from the group consisting of a bond, —NH—, —N(C1-4alkyl)-, —O—, —C(═O)—, —NH—C(═O)—, —N(C1-4alkyl)-C(═O)—, —C(═O)—NH—, —C(═O)—N(C1-4alkyl)-, —C(═O)—, C1-6alkylen, C3-7cycloalkylene, phenylene, 4-12 membered heterocyclylene and 5-10 membered heteroarylene;
wherein each C1-6alkylen, C3-7cycloalkylene, phenylene, 4-12 membered heterocyclylene and 5-10 membered heteroarylene in L1, L2 and L3 is optionally and independently substituted with one or more, identical or different substituent(s) selected from the group consisting of C2-6alkinyl, C1-6haloalkyl, C3-7cycloalkyl, phenyl, 5-6 membered heteroaryl, halogen, —OH, —CN, C1-6alkoxy, —NH2, —NH(C1-4alkyl), —N(C1-4alkyl)2, —C(═O)OH, —C(═O)—OC1-6alkyl, —C(═O)NH2, —C(═O)NH(C1-4alkyl), —C(═O)N(C1-4alkyl)2, the bivalent substituent ═O and C1-6alkyl optionally substituted with one or more, identical or different substituent(s) selected from the group consisting of halogen, —OH, —CN, C1-4alkoxy, —NH2, —NH(C1-4alkyl), —N(C1-4alkyl)2, —C(═O)OH, —C(═O)—OC1-6alkyl, —C(═O)NH2, —C(═O)NH(C1-4alkyl) and —C(═O)N(C1-4alkyl)2;
E is
 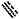 represents a double or a triple bond; represents a double or a triple bond;Q1 is selected from the group consisting of a bond, —CH2—, —CH(OH)—, —C(═O)—, —C(═O)N(RG1)—, —C(═O)O—, —S(═O)2—, —S(═O)2N(RG1)— and —C(═NRH1)—;
to each RG1 is independently selected from the group consisting of hydrogen, C1-6alkyl, C1-6haloalkyl, hydroxy-C1-6alkyl, H2N—C1-6alkyl, cyano-C1-6alkyl, (C1-4alkyl)HN—C1-6alkyl, (C1-4alkyl)2N—C1-6alkyl, C1-6alkoxy-C1-6alkyl, C3-7cycloalkyl and 3-11 membered heterocyclyl;
each RH1 is independently selected from the group consisting of hydrogen, —OH, C1-6alkoxy, —CN and C1-6alkyl;
if
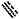 represents a double bond then represents a double bond thenRD is selected from the group consisting of hydrogen, C3-7cycloalkyl, phenyl, halogen, —CN, C1-6alkoxy, —C(═O)O—C1-6alkyl, —NHC(═O)—C1-6alkyl and C1-6alkyl optionally substituted with one or more, identical or different substituent(s) selected from the group consisting of phenyl, 3-11 membered heterocyclyl, C1-6alkoxy, halogen, —OH, —NH2, —NH(C1-6alkyl), —N(C1-6alkyl)2, —C(═O)OH, —C(═O)O—C1-6alkyl, —C(═O)NH(C1-6alkyl), —NHC(═O)—C1-6alkyl, —OC(═O)—C1-6alkyl and phenyl-C1-6alkoxy;
RE and RF is each independently selected from the group consisting of Ra2 and Rb2;
Ra2 is selected from the group consisting of hydrogen, C1-6alkyl, C1-6haloalkyl, C3-10cycloalkyl, 3-11 membered heterocyclyl, C6-10aryl and 5-10 membered heteroaryl, wherein the C1-6alkyl, C1-6haloalkyl, C3-10cycloalkyl, 3-11 membered heterocyclyl, C6-10aryl and 5-10 membered heteroaryl are all optionally substituted with one or more, identical or different Rb2 and/or Rc2;
each Rb2 is independently selected from the group consisting of —ORc2, —NRc2Rc2, halogen, —CN, —C(═O)Rc2, —C(═O)ORc2, —C(═O)NRc2Rc2, —S(═O)2Rc2, —S(═O)2NRc2Rc2, —NHC(═O)Rc2, —N(C1-4alkyl)C(═O)Rc2, —NHC(═O)ORc2, —N(C1-4alkyl)C(═O)ORc2 and the bivalent substituent ═O;
each Rc2 is independently selected from the group consisting of hydrogen, C1-6alkyl, C1-6haloalkyl, C2-6alkenyl, C2-6alkynyl, C3-10cycloalkyl, C4-10cycloalkenyl, 3-11 membered heterocyclyl, C6-10aryl and 5-10 membered heteroaryl, wherein the C1-6alkyl, C1-6haloalkyl, C2-6alkenyl, C2-6alkynyl, C3-10cycloalkyl, C4-10cycloalkenyl, 3-11 membered heterocyclyl, C6-10aryl and 5-10 membered heteroaryl are all optionally substituted with one or more, identical or different substituent(s) selected from the group consisting of C1-6alkyl, C1-6alkoxy, halogen, —OH, —C(═O)OH, —C(═O)O—C1-6alkyl, —C(═O)C1-6alkyl, —C(═O)NH2, —C(═O)NH(C1-6alkyl), —C(═O)N(C1-6alkyl)2, and the bivalent substituent ═O;
or
RD and RE taken together with the carbon atoms they are attached form a 4-7 membered unsaturated alicycle or 4-7 membered unsaturated heterocycle, wherein this 4-7 membered unsaturated alicycle or 4-7 membered unsaturated heterocycle is optionally, in addition to RF, substituted with one or more identical or different substituent(s) selected from the group consisting of C1-6alkyl, C1-6haloalkyl, —OH, C1-6alkoxy, C1-4alkoxy-C1-4alkyl, —NH2, —CN, —NH(C1-4alkyl), —N(C1-4alkyl)2, halogen, —C(═O)O—C1-6alkyl and the bivalent substituent ═O;
or
if Q1 is —C(═O)N(RG1)—, then RG1 of —C(═O)N(RG1)— and RF together form a linker selected from the group consisting of —C(═O)—, —CH2—, —CH2—C(═O)—, —C(═O)—CH2— and —C2H4—;
if
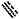 represents a triple bond then represents a triple bond thenRD and RE are both absent;
RF is Ra2;
Ra2 is selected from the group consisting of hydrogen, C1-6alkyl, C1-6haloalkyl, C3-10cycloalkyl, 3-11 membered heterocyclyl, C6-10aryl and 5-10 membered heteroaryl, wherein the C1-6alkyl, C1-6haloalkyl, C3-10cycloalkyl, 3-11 membered heterocyclyl, C6-10aryl and 5-10 membered heteroaryl are all optionally substituted with one or more, identical or different Rb2 and/or Rc2;
each Rb2 is independently selected from the group consisting of —ORc2, —NRc2Rc2, halogen, —CN, —C(═O)Rc2, —C(═O)ORc2, —C(═O)NRc2Rc2, —S(═O)2Rc2, —S(═O)2NRc2Rc2, —NHC(═O)Rc2, —N(C1-4alkyl)C(═O)Rc2, —NHC(═O)ORc2, —N(C1-4alkyl)C(═O)ORc2 and the bivalent substituent ═O;
each Rc2 is independently selected from the group consisting of hydrogen, C1-6alkyl, C1-6haloalkyl, C3-10cycloalkyl, 3-11 membered heterocyclyl, C6-10aryl and 5-10 membered heteroaryl;
or
 Q2 is selected from the group consisting of a bond, —CH2—, —CH(OH)—, —C(═O)—, —C(═O)N(RG2)—, —C(═O)O—, —S(═O)2—, —S(═O)2N(RG2)— and —C(═NRH2)—;
each RG2 is independently selected from the group consisting of hydrogen, C1-6alkyl, C1-6haloalkyl, hydroxy-C1-6alkyl, H2N—C1-6alkyl, cyano-C1-6alkyl, (C1-4alkyl)HN—C1-6alkyl, (C1-4alkyl)2N—C1-6alkyl, C1-6alkoxy-C1-6alkyl, C3-7cycloalkyl and 3-11 membered heterocyclyl;
each RH2 is independently selected from the group consisting of hydrogen, —OH, C1-6alkoxy, —CN and C1-6alkyl;
RI is selected from the group consisting of hydrogen and halogen;
RJ is hydrogen; or
RI and RJ together with the carbon atoms they are attached form a cyclopropane or oxirane ring;
RK is selected from the group consisting of hydrogen, C1-6alkyl, —CN and halogen;
RL is selected from the group consisting of hydrogen, C1-6alkyl, —CN, halogen and —C(═O)—C1-6alkyl;
or
E is
 Q3 is selected from the group consisting of —C(═O)—, —C(═O)N(RG3)—, —C(═O)O—, —S(═O)2—, —S(═O)2N(RG3)— and —C(═NRH3)—;
each RG3 is independently selected from the group consisting of hydrogen, C1-6alkyl, C1-6haloalkyl, hydroxy-C1-6alkyl, H2N—C1-6alkyl, cyano-C1-6alkyl, (C1-4alkyl)HN—C1-6alkyl, (C1-4alkyl)2N—C1-6alkyl, C1-6alkoxy-C1-6alkyl, C3-7cycloalkyl and 3-11 membered heterocyclyl;
each RH3 is independently selected from the group consisting of hydrogen, —OH, C1-6alkoxy, —CN and C1-6alkyl;
RM is selected from the group consisting of halogen, —CN and —O—C(═O)—C1-6alkyl;
or
E is
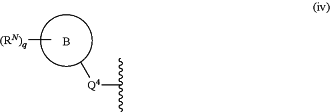 Q4 is selected from the group consisting of a bond, —C(═O)—, —C(═O)O—, —C(═O)NH—, —C(═O)N(C1-4alkyl)-, —S(═O)2— and —S(═O)2NH—;
ring B is selected from the group consisting of phenyl, pyridyl, pyrimidyl, pyridazinyl, pyrazinyl and 5-membered heteroaryl;
q is selected from the group consisting 1, 2, 3 and 4;
each RN is independently selected from the group consisting of C1-4alkyl, C1-4haloalkyl, vinyl, ethinyl, halogen, —CN, nitro and C1-4alkoxy;
or a salt thereof.
|