| CPC C07D 401/14 (2013.01) [A61K 45/06 (2013.01); C07D 401/06 (2013.01); C07D 405/14 (2013.01); C07D 409/14 (2013.01); C07D 417/14 (2013.01); C07B 2200/05 (2013.01)] | 66 Claims |
|
1. A compound of Formula Ib:
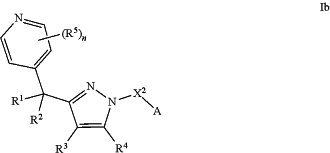 or a pharmaceutically acceptable salt thereof, wherein:
X2 is —(CR6R7)m or —(CR6R7)p—C(═O)—(CR6R7)q—;
A is halo, CN, Cy, or C1-3 haloalkyl;
R1, R2, R3, R4, and R5 are each independently selected from H, D, halo, CH3, CH2CH3, CD3, CH2CD3, and CD2CD3;
R6 and R7 are each independently selected from H, D, halo, methyl, ethyl, and C1-3 haloalkyl;
Cy is selected from C6-10 aryl, C3-7 cycloalkyl, 5-6 membered heteroaryl, and 4-7 membered heterocycloalkyl, each optionally substituted by 1, 2, 3, 4, or 5 RCy substituents independently selected from halo, C1-4 alkyl, C1-4 haloalkyl, CN, NO2, ORa, SRa, C(O)Rb, C(O)NRcRd, C(O)ORa, OC(O)Rb, OC(O)NRcRd, NRcRd, NRcC(O)Rb, NRcC(O)ORa, NRcC(O)NRcRd, C(═NRe)Rb, C(═NRe)NRcRd, NRcC(═NRe)NRcRd, NRCS(O)Rb, NRCS(O)2Rb, NRCS(O)2NRcRd, S(O)Rb, S(O)NRcRd, S(O)2Rb, and S(O)2NRcRd;
or two adjacent RCy substituents together with the atoms to which they are attached form a fused phenyl, C3-7 cycloalkyl, 5-6 membered heteroaryl, or 4-7 membered heterocycloalkyl ring, each optionally substituted by 1, 2, 3, 4, or 5 substituents independently selected from halo, C1-4 alkyl, C1-4 haloalkyl, CN, NO2, ORa, SRa, C(O)Rb, C(O)NRcRd, C(O)ORa, OC(O)Rb, OC(O)NRcRd, NRcRd, NRcC(O)Rb, NRcC(O)ORa, NRcC(O)NRcRd, C(═NRe)Rb, C(═NRe)NRcRd, NRcC(═NRe)NRcRd, NRcS(O)Rb, NRcS(O)2Rb, NRCS(O)2NRcRd, S(O)Rb, S(O)NRcRd, S(O)2Rb, and S(O)2NRcRd;
each Ra, Rb, Rc, and Rd is independently selected from H, C1-4 alkyl, and C1-4 haloalkyl, wherein said C1-4 alkyl is optionally substituted with 1, 2, or 3 substituents independently selected from OH, CN, amino, halo, C1-4 alkyl, C1-4 alkoxy, C1-4 haloalkyl, and C1-4 haloalkoxy;
each Re is independently selected from H, C1-4 alkyl, and CN;
m is 1, 2, or 3;
n is 0, 1, or 2;
p is 0, 1, or 2; and
q is 0, 1, or 2.
|