| CPC C07D 239/42 (2013.01) [C07D 213/76 (2013.01); C07D 401/06 (2013.01); C07D 403/06 (2013.01)] | 22 Claims |
|
1. A compound of Formula (I), or a pharmaceutically acceptable salt, or solvate thereof:
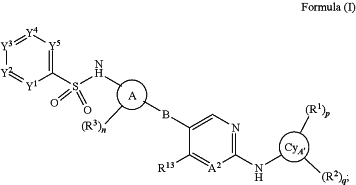 wherein
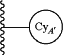 is C3-C10cycloalkyl;
each R1 is independently —OR6a, —SR6a, —S(═O)R7, —S(═O)2R7, or —N(R6b)2;
each R2 is independently halogen, —CN, —OR8a, —SR8a, —S(═O)R9, —S(═O)2R9, —S(═O)2N(R8b)2, —NR8aS(═O)2R9, —C(═O)R9, —OC(═O)R9, —CO2R8a, —OCO2R9, —N(R8b)2, —OC(═O)N(R8b)2, —NR8aC(═O)R9, —NR8aC(═O)OR9, optionally substituted C1-C4alkyl, optionally substituted C1-C4fluoroalkyl, optionally substituted C1-C4heteroalkyl, optionally substituted C3-C6cycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl;
Y5 is CR4;
Y1, Y2, Y3, and Y4 are each independently selected from N and CR5 with the proviso that no more than two of Y1, Y2, Y3, and Y4 are N;
each R6a is independently H, optionally substituted C1-C4alkyl, optionally substituted C1-C4heteroalkyl, optionally substituted C1-C4fluoroalkyl, —X-optionally substituted C1-C4alkyl, —X-optionally substituted C1-C4heteroalkyl, —X-optionally substituted C1-C4fluoroalkyl, optionally substituted C3-C6cycloalkyl, optionally substituted C3-C4cycloalkylC1-C3alkyl, optionally substituted C2-C10heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl;
each R6b is independently H, optionally substituted C1-C4alkyl, optionally substituted C1-C4heteroalkyl, optionally substituted C1-C4fluoroalkyl, —X-optionally substituted C1-C4alkyl, —X-optionally substituted C1-C4heteroalkyl, —X-optionally substituted C1-C4fluoroalkyl, optionally substituted C3-C6cycloalkyl, optionally substituted C3-C4cycloalkylC1-C3alkyl, optionally substituted C2-C10heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl;
or two R6b are taken together with the N atom to which they are attached to form an optionally substituted heterocycle;
X is —C(═O)—;
each R7 is independently optionally substituted C1-C4alkyl, optionally substituted C1-C4heteroalkyl, optionally substituted C1-C4fluoroalkyl, optionally substituted C3-C6cycloalkyl, optionally substituted C2-C10heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl;
each R8a is independently H, optionally substituted C1-C4alkyl, optionally substituted C1-C4heteroalkyl, optionally substituted C1-C4fluoroalkyl, optionally substituted C3-C6cycloalkyl, optionally substituted C2-C10heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl;
each R8b is independently H, optionally substituted C1-C4alkyl, optionally substituted C1-C4heteroalkyl, optionally substituted C1-C4fluoroalkyl, optionally substituted C3-C6cycloalkyl, optionally substituted C2-C10heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl;
or two R8b are taken together with the N atom to which they are attached to form an optionally substituted heterocycle;
each R9 is independently optionally substituted C1-C4alkyl, optionally substituted C1-C4heteroalkyl, or optionally substituted C1-C4fluoroalkyl, optionally substituted C1-C4cycloalkyl, optionally substituted C2-C10heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl;
A2 is N or CRA;
RA is H, halogen, optionally substituted C1-C4alkyl, optionally substituted C1-C4fluoroalkyl, optionally substituted aryl, or —OR10;
RA1, RA2, RA3 and RA4 are each independently H, optionally substituted C1-C4alkyl, optionally substituted C1-C4fluoroalkyl, or optionally substituted aryl;
R10 is independently H, optionally substituted C1-C4alkyl, optionally substituted C1-C4fluoroalkyl, optionally substituted C1-C4heteroalkyl, optionally substituted C3-C6cycloalkyl, optionally substituted C2-C10heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl;
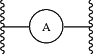 is phenylene or a 6-membered heteroarylene ring comprising 1 or 2 nitrogen atoms in the ring;
B is
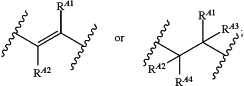 each R3 is independently halogen, —CN, —OR11, —SR11, —N(R11)2, optionally substituted C1-C4alkyl, optionally substituted C1-C4fluoroalkyl, optionally substituted C1-C4heteroalkyl, optionally substituted C3-C6cycloalkyl, optionally substituted C2-C10heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl;
each R11 is independently H, optionally substituted C1-C4alkyl, optionally substituted C1-C4fluoroalkyl, optionally substituted C1-C4heteroalkyl, optionally substituted C3-C6cycloalkyl, optionally substituted C2-C10heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl;
n is 0, 1, 2, 3, or 4;
p is 1, 2, or 3;
q is 0, 1, 2, or 3;
R4 and each R5 are each independently H, halogen, —CN, —OR12, —SR12, —N(R12)2, optionally substituted C1-C4alkyl, optionally substituted C1-C4fluoroalkyl, optionally substituted C1-C4heteroalkyl, optionally substituted C3-C6cycloalkyl, optionally substituted C2-C10 heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl; and
R12 is independently H, optionally substituted C1-C4alkyl, optionally substituted C1-C4fluoroalkyl, optionally substituted C1-C4heteroalkyl, optionally substituted C3-C6cycloalkyl, optionally substituted C2-C10heterocycloalkyl, optionally substituted aryl, or optionally substituted heteroaryl.
|