| CPC C07D 215/06 (2013.01) [C07C 5/02 (2013.01); C07D 215/04 (2013.01); C07D 215/12 (2013.01); C07D 215/18 (2013.01); C07D 221/06 (2013.01); C07D 221/12 (2013.01); C07D 401/14 (2013.01); C08G 18/7862 (2013.01); C08G 73/0688 (2013.01); C08L 79/02 (2013.01); C01B 32/152 (2017.08); C01B 32/182 (2017.08)] | 42 Claims |
|
1. A method of producing biquinolines or polyquinolines comprising:
reacting a bifunctional aromatic aldimine with an aromatic mono- or bi-alkynyl in the presence of a Lewis acid mediator and an oxidant to produce the corresponding biquinoline or polyquinoline, wherein:
the bifunctional aromatic aldimine is of formula:
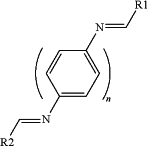 comprising:
a first aromatic core comprising aromatic or heteroaromatic rings, wherein
n is a number of distinct rings and is an integer of at least 1, and
the first aromatic core is substituted with at least one halogen;
a first aldimine functionality immediately and covalently attached to the first aromatic core, wherein
R1 is an aromatic or heteroaromatic functionality optionally substituted with one or more substituents, each independently selected from the group consisting of: a halogen, an alkyl, an alkoxy, an acetyl, an N-acetyl, an amine, an alkyl amine, a sulfide, a nitrate, a nitrile, another electron withdrawing or electron donating functionality, or any combination thereof; and
a second aldimine functionality immediately and covalently attached to the first aromatic core, wherein
R2 is one of the functionalities selected from the group consisting of: R1, a substituted aromatic ring, an unsubstituted aromatic ring, a substituted heteroaromatic ring, and an unsubstituted heteroaromatic ring; and wherein
the at least one halogen is at meta position of the first aromatic core relative to the first aldimine functionality; and
the aromatic mono- or bi-alkynyl comprises a second aromatic core and one or two aromatic terminal alkyne functionalities respectively; and wherein
the first and the second aldimine functionalities of the bifunctional aromatic aldimine react with the one or two aromatic terminal alkyne functionalities of the aromatic mono- or bi-alkynyl to yield the corresponding biquinoline or polyquinoline comprising quinoline moieties incorporating the nitrogens of the first and the second aldimine functionalities.
|