| CPC A61N 1/0534 (2013.01) [A61N 1/0531 (2013.01); A61N 1/36082 (2013.01); A61B 5/291 (2021.01); A61N 1/36064 (2013.01); A61N 1/36171 (2013.01)] | 25 Claims |
|
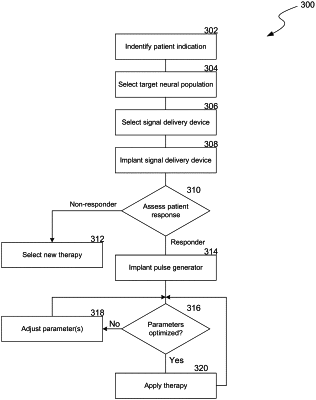
1. A method for treating a patient, comprising:
programming an implantable signal generator to deliver an electrical signal to a target neural population in the patient's brain, via an implanted signal delivery device, to reduce or eliminate the effects of a patient disorder and without the electrical signal inducing paresthesia,
wherein the electrical signal has a frequency in a frequency range of from 5 kHz to 500 kHz, an amplitude in an amplitude range of from 0.1 mA to 20 mA, and a pulse width in a pulse width range of from 10 microseconds to 333 microseconds.
|