| CPC A61M 5/2459 (2013.01) [A61M 5/34 (2013.01); A61M 39/24 (2013.01); A61M 2005/2006 (2013.01); A61M 2005/3103 (2013.01); A61M 2005/3117 (2013.01); A61M 2005/3123 (2013.01); A61M 2005/3128 (2013.01)] | 15 Claims |
|
1. A multi-use drug delivery device for extended use, wherein the multi-use drug delivery device comprises a multi-use main portion comprising a central axis (A) defining a longitudinal direction and a single-use flow conducting device adapted for conducting drug to subcutaneous tissue of a subject, wherein the drug delivery device is adapted to enable the flow conducting device to be movably arranged relative to the main portion, wherein the relative movement can be guided by cooperating structures of the main portion and the flow conducting device, wherein the drug delivery device is configurable in:
a connected configuration, wherein the flow conducting device is arranged in a connected position relative to the main portion, wherein the relative movement is guided, and wherein the relative position defines a first distance (d1) in the longitudinal direction between the flow conducting device and the main portion, wherein the relative position corresponds to a first internal volume confined by an outer surface of the drug delivery device,
an intermediate configuration, wherein the flow conducting device is movably arranged in an intermediate position relative to the main portion, wherein the relative movement is guided, and wherein the relative position defines a second distance (d2) in the longitudinal direction between the flow conducting device and the main portion, which is larger than the first distance (d1), wherein the relative position corresponds to a second internal volume confined by an outer surface of the drug delivery device,
an unconnected configuration, wherein the flow conducting device is arranged in an unconnected position relative to the main portion, wherein the relative movement is not guided, and thereby the main portion and the flow conducting device are not connected, and
wherein a change of configuration of the drug delivery device is adapted to be provided by a movement of the flow conducting device in the longitudinal direction relative to the main portion, wherein the change from the connected configuration to the unconnected configuration is through the intermediate configuration,
wherein the second internal volume is larger than the first internal volume, and whereby the internal volume which transitions from the first internal volume to the second internal volume and confined by the outer surface of the drug delivery device expands, in response to changing the drug delivery device from the connected configuration to the intermediate configuration,
wherein the main portion of the drug delivery device comprises a drug reservoir, a drug expelling mechanism for pressurizing the reservoir and thereby expelling an amount of drug, wherein the reservoir comprises multiple doses of a liquid drug formulation, and wherein the drug formulation allows microbial growth upon introduction of microorganisms into the reservoir during extended use,
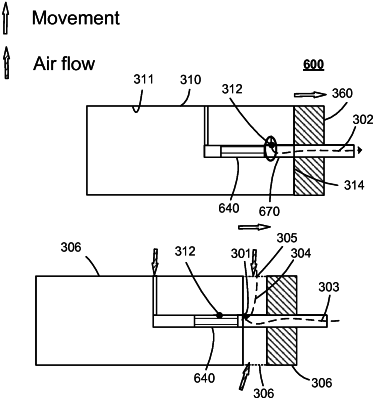
wherein the main portion further comprises a drug outlet and an outlet surface, wherein the outlet surface provides a portion of an outer surface of the main portion, and
wherein the flow conducting device comprises a flow channel comprising a channel inlet and a channel outlet, wherein the flow channel is adapted for forming a combined flow path with the drug outlet,
wherein the flow conducting device further comprises an inlet surface for interfacing the outlet surface of the main portion,
wherein the drug delivery device further defines a fluid communication volume being a fluid or gaseous volume at the channel inlet, and an unintended flow path from the channel outlet through the flow channel and to the fluid communication volume, and
wherein the connected configuration further comprises, the outlet surface is interfacing the inlet surface, and wherein the drug outlet is arranged in the outlet surface to allow the drug to flow from the main portion to the flow conducting device along the combined flow path, in response to the pressure in the reservoir exceeding a pressure threshold, and wherein the fluid communication volume provides a portion of the combined flow path and the first internal volume of the drug delivery device, and
wherein the unconnected configuration further comprises, the outlet surface is exposed to the external environment, the drug outlet is arranged in a closed state, and wherein the drug outlet is arranged to inhibit the introduction of microorganisms from the external environment and into the reservoir,
wherein the intermediate configuration further comprises an equalizing channel defining an equalizing flow path different from the unintended flow path, wherein the equalizing channel defining the equalizing flow path is adapted for equalizing the fluid pressure in the fluid communication volume to the fluid pressure at the channel outlet, upon changing the configuration from the connected to the intermediate configuration and thereby expanding an internal volume which transitions from the first internal volume to the second internal volume, whereby an equalizing fluid flow along the equalizing flow path is larger than an unintended flow along the unintended flow path, whereby equalization of the fluid communication volume is provided with a reduced risk of introducing microorganisms from the flow channel into the reservoir.
|