| CPC A61K 31/4439 (2013.01) [A61K 9/1605 (2013.01); A61K 9/4858 (2013.01); A61K 9/4866 (2013.01)] | 23 Claims |
|
1. A capsule dosage form comprising:
(i) from about 65% to about 93% w/w of Compound 1;
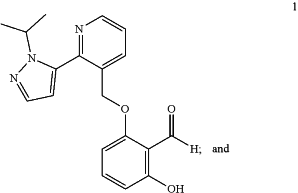 (ii) from about 2% to about 10% w/w a binder;
wherein w/w is relative to the total weight of the formulation, excluding the weight of the capsule, and
wherein the compound 1 is in a crystalline ansolvate form that is characterized by at least four X-ray powder diffraction peaks (Cu Kα radiation) at 13.37°, 14.37°, 19.95° and 23.92° 2θ, each peak is ±0.2° 2θ.
|