| CPC A61K 31/4439 (2013.01) [A61K 9/0019 (2013.01); A61K 9/0053 (2013.01); A61K 9/4825 (2013.01); A61K 31/34 (2013.01); A61K 31/4418 (2013.01); A61K 31/536 (2013.01); A61K 31/5365 (2013.01); A61K 31/685 (2013.01); A61P 31/18 (2018.01)] | 34 Claims |
|
1. A method of treating human immunodeficiency virus (HIV) infection in a heavily treatment-experienced patient, the method comprising administering to the patient a therapeutically effective amount of a pharmaceutical composition comprising a sodium salt of the compound of Formula (Ia):
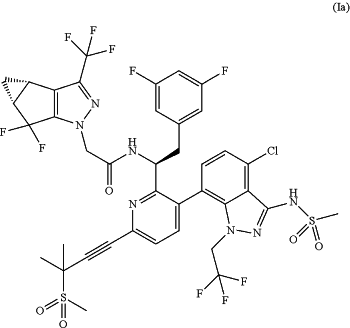 one or more pharmaceutically acceptable excipients, wherein the pharmaceutical composition is in the form of a solution comprising about 10 w/w % to about 40 w/w % water, about 35 w/w % to about 75 w/w % polyethylene glycol 300 (PEG 300), and about 5 w/w % to about 35 w/w % of the sodium salt of the compound of Formula (Ia);
wherein the HIV infection is an HIV-1 infection characterized by HIV-1 mutant resistance to one or more antiretroviral medications.
|