| CPC A61B 17/105 (2013.01) [A61B 17/00491 (2013.01); A61B 17/0644 (2013.01); A61B 17/068 (2013.01); A61B 17/072 (2013.01); A61B 17/07207 (2013.01); A61B 17/07292 (2013.01); A61B 17/08 (2013.01); A61B 17/32 (2013.01); A61L 17/105 (2013.01); A61L 17/12 (2013.01); B05D 1/007 (2013.01); B05D 1/30 (2013.01); B29C 48/16 (2019.02); B32B 3/02 (2013.01); B32B 3/08 (2013.01); B32B 3/20 (2013.01); B32B 3/266 (2013.01); B32B 5/024 (2013.01); B32B 5/026 (2013.01); B32B 5/10 (2013.01); B32B 5/12 (2013.01); B32B 5/18 (2013.01); B32B 5/245 (2013.01); B32B 5/26 (2013.01); B32B 7/05 (2019.01); D01D 5/0023 (2013.01); D01D 5/18 (2013.01); D04H 1/565 (2013.01); A61B 2017/00004 (2013.01); A61B 2017/00477 (2013.01); A61B 2017/00526 (2013.01); A61B 2017/00831 (2013.01); A61B 2017/00862 (2013.01); A61B 2017/00893 (2013.01); A61B 2017/00955 (2013.01); A61B 2017/00964 (2013.01); A61B 2017/07214 (2013.01); A61B 2017/07221 (2013.01); A61B 2017/07228 (2013.01); A61B 2017/07235 (2013.01); A61B 2017/07242 (2013.01); A61B 2017/0725 (2013.01); A61B 2017/07257 (2013.01); A61B 2017/07271 (2013.01); A61B 2017/07278 (2013.01); A61B 2017/07285 (2013.01); A61L 2420/02 (2013.01); B29C 44/3453 (2013.01); B29C 44/358 (2013.01); B29C 48/0012 (2019.02); B29C 48/0022 (2019.02); B29C 48/05 (2019.02); B29L 2031/753 (2013.01); B29L 2031/7546 (2013.01); B32B 2250/03 (2013.01); B32B 2250/40 (2013.01); B32B 2307/50 (2013.01); B32B 2307/54 (2013.01); B32B 2307/72 (2013.01); B32B 2307/732 (2013.01); B32B 2307/736 (2013.01); B32B 2535/00 (2013.01); D10B 2331/041 (2013.01); D10B 2509/00 (2013.01)] | 20 Claims |
|
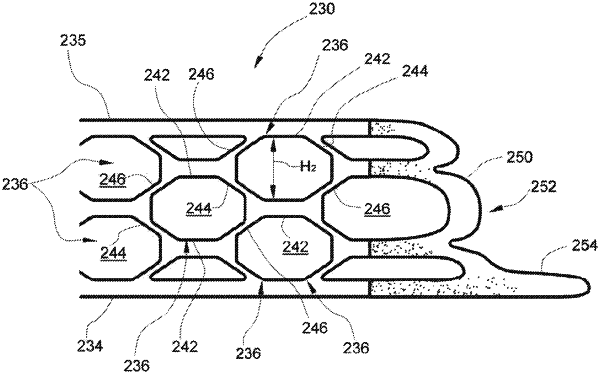
1. An adjunct, comprising:
a first layer to interface with a staple cartridge;
a second layer to interface with tissue, wherein the first layer and the second layer are configurable between:
an uncompressed state in which a first space is defined between the first layer and the second layer; and
a compressed state in which a second space less than the first space is defined between the first layer and the second layer; and
a support layer intermediate the first layer and the second layer, wherein the support layer comprises walls that define cavities within the support layer, and wherein the cavities are interconnected;
wherein the adjunct comprises a polymeric composition that comprises a pharmaceutical agent, wherein the pharmaceutical agent is to be released from the polymeric composition to effect tissue healing at a faster rate than without the pharmaceutical agent.
|