| CPC A01N 53/00 (2013.01) [C07C 237/42 (2013.01); C07C 237/52 (2013.01); C07C 243/38 (2013.01); C07C 255/19 (2013.01); C07C 255/46 (2013.01); C07C 255/58 (2013.01); C07C 255/60 (2013.01); C07C 271/18 (2013.01); C07C 271/22 (2013.01); C07C 271/28 (2013.01); C07C 271/66 (2013.01); C07C 281/02 (2013.01); C07C 281/06 (2013.01); C07C 311/09 (2013.01); C07C 311/10 (2013.01); C07C 311/21 (2013.01); C07C 311/48 (2013.01); C07C 317/24 (2013.01); C07C 337/06 (2013.01); C07C 381/00 (2013.01); C07D 213/77 (2013.01); C07D 213/81 (2013.01); C07D 237/20 (2013.01); C07D 239/42 (2013.01); C07D 307/68 (2013.01); C07D 333/38 (2013.01); C07C 2601/02 (2017.05); C07C 2601/04 (2017.05); C07C 2601/08 (2017.05); C07C 2601/16 (2017.05)] | 23 Claims |
|
1. A composition comprising:
(a) a molecule having the following formula
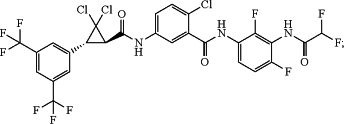 (b) an active ingredient selected from the group consisting of Abamectin, Acephate, Acetamiprid, Afidopyropen, Bifenthrin, Chlorantraniliprole, Chlorfenapyr, Cyantraniliprole, Dinotefuran, Emamectin Benzoate, Ethiprole, Fluxametamide, Imidacloprid, Lambda Cyhalothrin, Methoxyfenozide, Oxamyl, Pyriproxyfen, Spinetoram, Spiromesifen, Spirotetramat, Sulfoxaflor, Thiamethoxam, and Triflumezopyrim,
wherein the weight ratio of said molecule to said active ingredient is 100:1 to 1:100.
|