| CPC C07D 498/04 (2013.01) [A61K 31/5383 (2013.01); A61P 15/00 (2018.01); C07B 2200/13 (2013.01)] | 23 Claims |
|
1. A method of treating vasomotor symptoms, insomnia associated with menopause, sleep disturbances associated with menopause, night time awakenings associated with menopause, or any combination thereof, comprising administering to a human in need thereof a pharmaceutically effective dose of 2-[3,5-Bis(trifluoromethyl)phenyl]-N-{4-(4-fluoro-2-methylphenyl)-6-[(7S,9aS)-7-(hydroxymethyl)hexahydropyrazino[2,1-c][1,4]oxazin-8(1H)-yl]-3-pyridinyl}-N,2-dimethylpropanamide (Compound A):
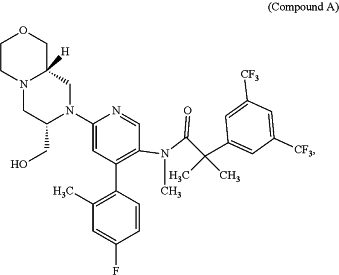 or a pharmaceutically acceptable salt thereof;
wherein compound A or a pharmaceutically acceptable salt thereof is administered in an oral form so as to reach pharmacokinetic (PK) exposures similar to that achieved when a soft gelatin capsule with a filling comprising:
(a) Compound A or a pharmaceutically acceptable salt thereof;
(b) at least one solubilizer selected from caprylocaproyl polyoxyl-8 glycerides, glycerol monocaprylocaprate, polyoxyl 35 castor oil, and polysorbate 80, or any mixtures thereof; and
(c) at least one antioxidant selected from DL-alpha-tocopherol, butylated hydroxytoluene, and butylated hydroxyanisole, or any mixtures thereof is administered.
|