| CPC C07D 471/16 (2013.01) [A61K 9/4825 (2013.01); A61K 9/4891 (2013.01); A61K 9/5031 (2013.01); A61K 31/4985 (2013.01); A61K 47/34 (2013.01); A61K 47/38 (2013.01); C07D 471/14 (2013.01)] | 28 Claims |
|
1. A pharmaceutical composition comprising a pharmaceutically acceptable diluent or carrier in admixture with:
(i) a compound of Formula Q:
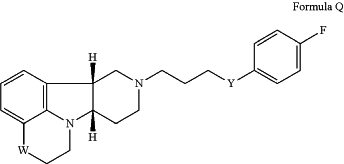 wherein:
W is —N(CH3)—; and
Y is —C(O)—;
in free base or pharmaceutically acceptable salt form; and
(ii) a polymeric matrix;
wherein:
(a) the compound of Formula Q is dissolved or dispersed in the pharmaceutical composition;
(b) the pharmaceutical composition is formulated as a sustained-release injectable composition or a delayed-release injectable composition;
(c) wherein the pharmaceutically acceptable diluent or carrier is an organic solvent; and
(d) wherein the pharmaceutical composition is non-aqueous.
|