| CPC C07D 267/14 (2013.01) [C07D 243/14 (2013.01); C07D 267/12 (2013.01); C07D 291/08 (2013.01); C07D 401/06 (2013.01); C07D 401/10 (2013.01); C07D 403/06 (2013.01); C07D 403/10 (2013.01); C07D 405/06 (2013.01); C07D 413/04 (2013.01); C07D 413/06 (2013.01); C07D 413/08 (2013.01); C07D 413/12 (2013.01); C07D 413/14 (2013.01); C07D 417/04 (2013.01); C07D 471/04 (2013.01); C07D 491/107 (2013.01); C07D 493/08 (2013.01); C07D 495/10 (2013.01); C07D 498/04 (2013.01); C07D 498/08 (2013.01)] | 17 Claims |
|
1. A method of treating breast cancer, lung cancer, ovarian cancer, or multiple myeloma in a subject in need thereof, comprising administering to the subject an effective amount of a compound is of Formula I:
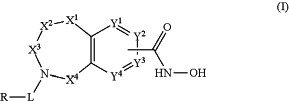 or a pharmaceutically acceptable salt thereof,
wherein:
X1 is O;
X2 and X4 are each CR1R2;
X3 is CR1′R2′;
Y1 and Y4 are not bonded to —C(O)NHOH and are CR1; Y2 and Y3 are each CR1 when not bonded to —C(O)NHOH, and Y2 and Y3 are C when bonded to —C(O)NHOH;
L is selected from the group consisting of —C(O)—, —C(O)(CR1R2)m—, and —C(O)(CR1R2)mO —, wherein L is bonded to the ring nitrogen through the carbonyl group;
R is independently, and at each occurrence, selected from the group consisting of —H, —C1-C6 alkyl, —C2-C6 alkenyl, —C4-C8 cycloalkenyl, —C2-C6 alkynyl, —C3-C8 cycloalkyl, —C5-C12 spirocyclyl, heterocyclyl, spiroheterocyclyl, aryl, and heteroaryl containing 1 to 5 heteroatoms selected from the group consisting of N, S, P, and O, wherein each alkyl, alkenyl, cycloalkenyl, alkynyl, cycloalkyl, spirocyclyl, heterocyclyl, spiroheterocyclyl, aryl, or heteroaryl is optionally substituted with one or more substituents selected from the group consisting of —OH, halogen, oxo, —NO2, CN, —R1, —R2, —OR3, —NHR3, —NR3R4, —S(O)2NR3R4, —S(O)2R1, —C(O)R1, —CO2R1, —NR3S(O)2R1, —S(O)R1, —S(O)NR3R4, —NR3S(O)R1, heterocyclyl, aryl, and heteroaryl containing 1 to 5 heteroatoms selected from the group consisting of N, S, P, and O, with the proviso that R is not bonded to L via a nitrogen atom;
each R1 and R2 is independently, at each occurrence, selected from the group consisting of —H, —R3, —R4, —C1-C6 alkyl, —C2-C6 alkenyl, —C4-C8 cycloalkenyl, —C2-C6 alkynyl, —C3-C8 cycloalkyl, heterocyclyl, aryl, heteroaryl containing 1 to 5 heteroatoms selected from the group consisting of N, S, P, and O, —OH, halogen, —NO2, —CN, —NHC1-C6 alkyl, —N(C1-C6 alkyl)2, —S(O)2N(C1-C6 alkyl)2, —N(C1-C6 alkyl)S(O)2R5, —S(O)2C1-C6 alkyl, —(C1-C6 alkyl)S(O)2R5, —C(O)C1-C6 alkyl, —CO2C1-C6 alkyl, —N(C1-C6 alkyl)S(O)2C1-C6 alkyl, and —(CHR5)nNR3R4, wherein each alkyl, alkenyl, cycloalkenyl, alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl is optionally substituted with one or more substituents selected from the group consisting of —OH, halogen, —NO2, oxo, —CN, —R5, —OR3, —NHR3, —NR3R4, —S(O)2N(R3)2, —S(O)2R5, —C(O)R5, —CO2R5, —NR3S(O)2R5, —S(O)R5, —S(O)NR3R4, —NR3S(O)R5, heterocyclyl, aryl, and heteroaryl containing 1 to 5 heteroatoms selected from the group consisting of N, S, P, and O;
or R1 and R2 can combine with the atom to which they are both attached to form a spirocycle, spiroheterocycle, or a spirocycloalkenyl;
or R1 and R2, when on adjacent atoms, can combine to form a heterocycle, cycloalkyl, cycloalkenyl, aryl, or heteroaryl containing 1 to 5 heteroatoms selected from the group consisting of N, S, P, and O;
or R1 and R2, when on non-adjacent atoms, can combine to form a bridging cycloalkyl, cycloalkenyl, or heterocycloalkyl;
each R1′ and R2′ is independently, at each occurrence, selected from the group consisting of H, aryl, and heteroaryl containing 1 to 5 heteroatoms selected from the group consisting of N, S, P, and O, wherein each aryl or heteroaryl is optionally substituted with one or more substituents selected from the group consisting of —OH, halogen, —NO2, oxo, —CN, —R3, —R5, —OR3, —NHR3, —NR3R4, —S(O)2N(R3)2, —S(O)2R5, —C(O)R5, —CO2R5, —NR3S(O)2R5, —S(O)R5, —S(O)NR3R4, —NR3S (O)R5, heterocycle, aryl, and heteroaryl containing 1 to 5 heteroatoms selected from the group consisting of N, S, P, and O, wherein at least one of R1′ or R2′ is not H;
each R3 and R4 is independently, at each occurrence, selected from the group consisting of —H, —C1-C6 alkyl, —C2-C6 alkenyl, —C4-C8 cycloalkenyl, —C2-C6 alkynyl, —C3-C8 cycloalkyl, heterocyclyl, aryl, heteroaryl containing 1 to 5 heteroatoms selected from the group consisting of N, S, P, and O, —S(O)2N(C1-C6 alkyl)2, —S(O)2C1-C6 alkyl, —(C1-C6 alkyl)S(O)2R5, —C(O)C1-C6 alkyl, —CO2C1-C6 alkyl, and —(CHR5)nN(C1-C6 alkyl)2, wherein each alkyl, alkenyl, cycloalkenyl, alkynyl, cycloalkyl, heterocyclyl, aryl, or heteroaryl is optionally substituted with one or more substituents selected from the group consisting of —OH, halogen, —NO2, oxo, —CN, —R5, —O(C1-C6) alkyl, —NHC1-C6 alkyl, N(C1-C6 alkyl)2, —S(O)2N(C1-C6 alkyl)2, —S(O)2NH(C1-C6 alkyl), —C(O)C1-C6 alkyl, —CO2C1-C6 alkyl, —N(C1-C6 alkyl)S(O)2C1-C6 alkyl, —S(O)R5, —S(O)N(C1-C6 alkyl)2, —N(C1-C6 alkyl)S(O)R5, heterocyclyl, aryl, and heteroaryl containing 1 to 5 heteroatoms selected from the group consisting of N, S, P, and O;
R5 is independently, at each occurrence, selected from the group consisting of —H, —C1-C6 alkyl, —C2-C6 alkenyl, —C4-C8 cycloalkenyl, —C2-C6 alkynyl, —C3-C8 cycloalkyl, heterocyclyl, aryl, heteroaryl containing 1 to 5 heteroatoms selected from the group consisting of N, S, P, and O, —OH, halogen, —NO2, —CN, —NHC1-C6 alkyl, —N(C1-C6 alkyl)2, —S(O)2NH(C1-C6 alkyl), —S(O)2N(C1-C6 alkyl)2, —S(O)2C1-C6 alkyl, —C(O)C1-C6 alkyl, —CO2C1-C6alkyl, —N(C1-C6alkyl)SO2C1-C6 alkyl, —S(O)(C1-C6 alkyl), —S(O)N(C1-C6 alkyl)2, —N(C1-C6 alkyl)S(O)(C1-C6 alkyl) and —(CH2)nN(C1-C6 alkyl)2;
each n is independently and at each occurrence an integer from 0 to 6; and
each m is independently and at each occurrence an integer from 1 to 6.
|