| CPC C01D 7/126 (2013.01) [C01D 7/24 (2013.01); C01D 7/26 (2013.01)] | 10 Claims |
|
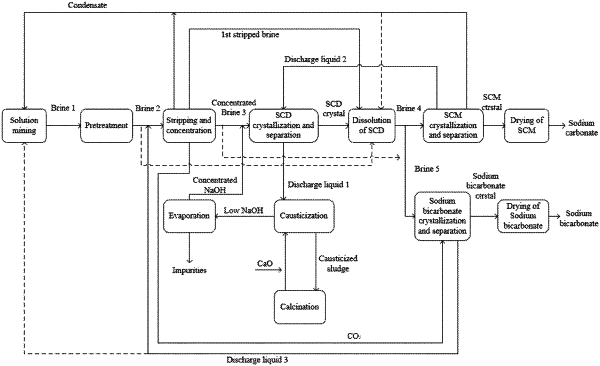
1. A soda ash and sodium bicarbonate production method, comprising:
(S1) solution mining
injecting water into trona ore to dissolve sodium carbonate and/or sodium bicarbonate to obtain a raw brine 1;
(S2) pretreatment
removing solid particles and/or total organic carbon (TOC) in the raw brine 1 to obtain a brine 2;
(S3) stripping and concentration
subjecting the brine 2 to stripping to convert most of sodium bicarbonate in the brine 2 to sodium carbonate and concentration to obtain a concentrated brine 3;
(S4) decahydrate crystallization and separation
subjecting the concentrated brine 3 to neutralization with a concentrated sodium hydroxide solution, crystallize at low-temperature in a sodium carbonate decahydrate crystallizer and separation to obtain a low liquid residual sodium carbonate decahydrate crystal and a discharge liquid 1;
(S5) dissolution of sodium carbonate decahydrate
dissolving the low liquid residual sodium carbonate decahydrate crystal to obtain a dissolved sodium carbonate decahydrate solution; and dividing the dissolved sodium carbonate decahydrate solution into two parts, respectively a brine 4 and a brine 5;
(S6) sodium carbonate monohydrate crystallization, separation and drying
subjecting the brine 4 to evaporation, concentration, crystallization in a sodium carbonate monohydrate crystallizer and separation to obtain a low liquid residual sodium carbonate monohydrate crystal and a discharge liquid 2; drying the low liquid residual sodium carbonate monohydrate crystal to obtain dense soda ash; mixing the discharge liquid 2 with the concentrated brine 3 to obtain a mixed solution; neutralizing the mixed solution; and feeding the mixed solution to the sodium carbonate decahydrate crystallizer followed by crystallization and separation;
(S7) sodium bicarbonate crystallization, separation and drying
subjecting the brine 5 to carbonation reaction with carbon dioxide in a sodium bicarbonate crystallizer, cooling, crystallization and separation to obtain a low liquid residual sodium bicarbonate crystal and a discharge liquid 3, wherein the discharge liquid 3 is collected to be used in step (S3) and/or step (S1); and drying the sodium bicarbonate crystal to obtain sodium bicarbonate;
(S8) causticization
subjecting the discharge liquid 1 and lime milk to causticization, followed by clarification and separation to obtain a low NaOH content solution and causticized sludge; and subjecting the causticized sludge to washing and calcination to obtain quick lime to be reused for causticization; and
(S9) evaporation
subjecting the NaOH solution to evaporation and concentration to remove impurities to obtain a concentrated NaOH solution, wherein the concentrated NaOH solution is used for neutralization of residual sodium bicarbonate in the concentrated brine 3 and discharge liquid 2.
|