| CPC A61K 9/0092 (2013.01) [A61K 9/0004 (2013.01); A61K 9/0034 (2013.01); A61K 31/167 (2013.01); A61M 31/002 (2013.01); A61M 2210/1085 (2013.01)] | 24 Claims |
|
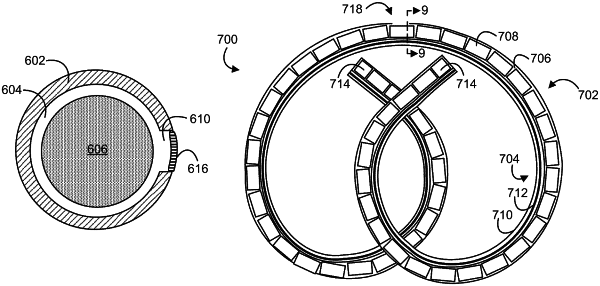
1. An intravesical device for drug delivery to the urinary bladder of a patient, comprising:
a tubular device body which comprises a drug reservoir lumen and a retention frame lumen;
a drug formulation comprising a plurality of aligned solid drug units, which comprise a drug, disposed in the drug reservoir lumen; and
a nitinol retention frame disposed in the retention frame lumen,
wherein the device has no drug release aperture and releases the drug by trans-wall diffusion; and
wherein the solid drug units are tablets each of which has a cylindrical side face and a length:width aspect ratio greater than 1:1.
|