| CPC H10K 85/6572 (2023.02) [C07D 209/08 (2013.01); C07D 209/56 (2013.01); C07D 209/80 (2013.01); C07D 209/82 (2013.01); C07D 209/86 (2013.01); C07D 209/88 (2013.01); C07D 215/06 (2013.01); C07D 219/08 (2013.01); C07D 219/14 (2013.01); C07D 241/48 (2013.01); C07D 265/38 (2013.01); C07D 279/02 (2013.01); C07D 279/22 (2013.01); C07D 279/26 (2013.01); C07D 279/34 (2013.01); C07D 403/04 (2013.01); C07D 409/10 (2013.01); C07D 409/14 (2013.01); C07D 417/10 (2013.01); C07D 417/14 (2013.01); C07D 487/04 (2013.01); C07F 7/0816 (2013.01); C09K 11/06 (2013.01); H10K 85/40 (2023.02); H10K 85/615 (2023.02); H10K 85/636 (2023.02); H10K 85/655 (2023.02); H10K 85/657 (2023.02); C09K 2211/1018 (2013.01); H10K 50/11 (2023.02); H10K 50/12 (2023.02); H10K 50/16 (2023.02); H10K 50/17 (2023.02); H10K 50/18 (2023.02); H10K 71/12 (2023.02); H10K 71/164 (2023.02); H10K 2101/30 (2023.02); H10K 2101/40 (2023.02); Y02E 10/549 (2013.01); Y02P 70/50 (2015.11)] | 13 Claims |
|
1. An optoelectronic device comprising at least one light-emitting layer,
wherein the at least one light emitting layer consists of an organic molecule comprising a structure of Formula I and a host material whose triplet (T1) and singlet (S1) energy levels are energetically higher than the triplet (T1) and singlet (S1) energy levels of the organic molecule,
wherein the organic molecule is a luminescent emitter,
wherein the at least one light emitting layer comprises no additional emitter material:
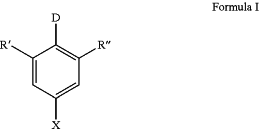 wherein
X=CN or CF3;
D=a chemical unit comprising a structure of Formula 1-1:
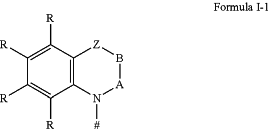 wherein
#=an attachment point of the unit of Formula I-1 to a central phenyl ring of the structure of Formula I;
A and B=independently of one another are selected from the group consisting of CRR1, CR, NR, and N, there being a single or double bond between A and B and a single or double bond between B and Z;
Z=a direct bond or a divalent organic bridge which is a substituted or unsubstituted C1 to C9-alkylene, C2 to C8-alkenylene, C2 to C8-alkynylene or arylene group or a combination thereof,
—C(Me)2, —C═CRR1, C═NR, —NR—, —O—, —SiRR1—, —S—, —S(O)—, —S(O)2—, O-interrupted substituted or unsubstituted C1 to C9-alkylene, C2 to C8-alkenylene, C2 to C8-alkynylene or arylene group, phenyl units or substituted phenyl units;
where each R and R1, identically or differently at each occurrence, is H, deuterium, azide (N3−), F, Cl, Br, I, N(R2)2, CN, CF3, NO2, OH, COOH, COOR2, CO (NR2)2, Si(R2)3, B(OR2)2, C(═O) R2, P(═O)(R2)2, S(═O)R2, S(═O)2R2, OSO2R2, a linear alkyl, alkoxy or thioalkoxy group having 1 to 40 carbon atoms or a linear alkenyl or alkynyl group having 2 to 40 carbon atoms or a branched or cyclic alkyl, alkenyl, alkynyl, alkoxy or thioalkoxy group having 3 to 40 carbon atoms, which in each case may be substituted by one or more radicals R2, it being possible for one or more non-adjacent CH2 groups to be replaced by R2C═CR2, C≡C, Si(R2)2, Ge(R2)2, Sn(R2)2, C═O, C═S, C═Se, C═NR2, P(═O)(R2), SO, SO2, NR2, O, S or CONR2, and it being possible for one or more H atoms to be replaced by deuterium, F, Cl, Br, I, CN, CF3 or NO2, or is an aromatic hydrocarbon ring system having 6 to 60 aromatic ring atoms optionally substituted by one or more radicals R2 or a heteroaromatic ring system having 5 to 60 aromatic ring atoms, or is an aryloxy or heteroaryloxy group having 5 to 60 aromatic ring atoms, wherein the aryloxy or the heteroaryloxy group is optionally substituted by one or more radicals R2, or is a diarylamino group, diheteroarylamino group or arylheteroarylamino group having 10 to 40 aromatic ring atoms, wherein the diarylamino group, the diheteroarylamino group or the arylheteroarylamino is optionally substituted by one or more radicals R2, or is a combination of these systems, or is a crosslinkable unit QE which may be crosslinked by acid-catalytic, thermal or UV crosslinking methods in the presence or absence of a photoinitiator or by microwave radiation; optionally two or more of these substituents R and R1 may form with one another a mono-or polycyclic, aliphatic, aromatic and/or benzo-fused ring system, wherein, when any R combines to form a carbazole group bonded to Formula I-1, the carbazole group is bonded to Formula I-1 through the nitrogen atom of the carbazole group;
R2, identically or differently at each occurrence, is H, deuterium, F, Cl, Br, I, N(R3)2, CN, CF3, NO2, OH, COOH, COOR3, CO(NR3)2, Si(R3)3, B(OR3)2, C(═O)R3, P(═O)(R3)2, S(═O)R3, S(═O)2R3, OSO2R3, a linear alkyl, alkoxy or thioalkoxy group having 1 to 40 carbon atoms or a linear alkenyl or alkynyl group having 2 to 40 carbon atoms or a branched or cyclic alkyl, alkenyl, alkynyl, alkoxy or thioalkoxy group having 3 to 40 C atoms, which may be substituted in each case by one or more radicals R3, it being possible for one or more non-adjacent CH2 groups to be replaced by R3C═CR3, C≡C, Si(R3)2, Ge(R3)2, Sn(R3)2, C═O, C═S, C═Se, C═NR3, P(═O)(R3), SO, SO2, NR3, O, S or CONR3, and it being possible for one or more H atoms to be replaced by deuterium, F, Cl, Br, I, CN, CF3 or NO2, or is an aromatic or heteroaromatic ring system having 5 to 60 aromatic ring atoms, wherein the aromatic or the heteroaromatic ring system is optionally substituted in each case by one or more radicals R3, or is an aryloxy or heteroaryloxy group having 5 to 60 aromatic ring atoms, wherein the aryloxy or the heteroaryloxy group is optionally substituted by one or more radicals R3, or is a diarylamino group, diheteroarylamino group or arylheteroarylamino group having 10 to 40 aromatic ring atoms, wherein the diarylamino group, the diheteroarylamino group or the arylheteroarylamino is optionally substituted by one or more radicals R3, or is a combination of these systems; where optionally two or more of these substituents R2 form with one another a mono-or polycyclic, aliphatic, aromatic and/or benzo-fused ring system;
R3, identically or differently at each occurrence, is H, deuterium, F, CF3 or an aliphatic, aromatic and/or heteroaromatic radical having 1 to 20 C atoms, in which also one or more H atoms may be replaced by F or CF3; where optionally two or more substituents R3 form with one another a mono-or polycyclic, aliphatic ring system;
R′=selected from the group consisting of H, N (R4)2, OR4, thiophene, a linear alkyl or alkoxy group having 1 to 40 C atoms and a branched or cyclic alkyl or alkoxy group having 3 to 40 carbon atoms, it being possible for this group to be substituted in each case by one or more radicals R4, and an aromatic hydrocarbon ring system having 6 to 60 aromatic ring atoms, wherein the aromatic ring system is optionally substituted in each case by one or more radicals R4;
R″=selected from the group consisting of N(R4)2, OR4, thiophene, a linear alkyl or alkoxy group having 1 to 40 C atoms and a branched or cyclic alkyl or alkoxy group having 3 to 40 carbon atoms, it being possible for this group to be substituted in each case by one or more radicals R4, and an aromatic hydrocarbon ring system having 6 to 60 aromatic ring atoms, wherein the aromatic ring system is optionally substituted by one or more radicals R4:
R4, identically or differently at each occurrence, is H, deuterium, N(R5)2, Si(R5)3, a linear alkyl, alkoxy or thioalkoxy group having 1 to 40 carbon atoms or a branched or cyclic alkyl, alkoxy or thioalkoxy group having 3 to 40 carbon atoms, it being possible for this group to be substituted in each case by one or more radicals R5, or is an aromatic or heteroaromatic ring system having 5 to 60 aromatic ring atoms, wherein the aromatic or the heteroaromatic ring system is optionally substituted in each case by one or more radicals R5, or is an aryloxy or heteroaryloxy group having 5 to 60 aromatic ring atoms, wherein the aryloxy or the heteroaryloxy group is optionally substituted by one or more radicals R5, or is a diarylamino group, diheteroarylamino group or arylheteroarylamino group having 10 to 40 aromatic ring atoms wherein the diarylamino group, the diheteroarylamino group or the arylheteroarylamino is optionally substituted by one or more radicals R5, or is a combination of these systems; where optionally two or more of these substituents R5 may also form with one another a mono-or polycyclic, aliphatic, aromatic and/or benzo-fused ring system; and
R5, identically or differently at each occurrence, is H, deuterium or an aliphatic, aromatic and/or heteroaromatic radical having 1 to 20 carbon atoms; where optionally two or more substituents R5 may also form with one another a mono-or polycyclic, aliphatic ring system.
|