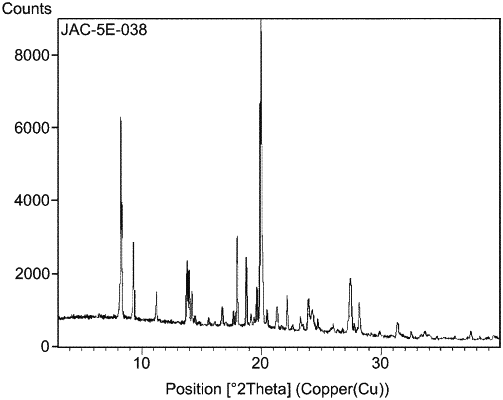
| CPC C07D 471/04 (2013.01) [C07B 2200/13 (2013.01)] | 16 Claims |
|
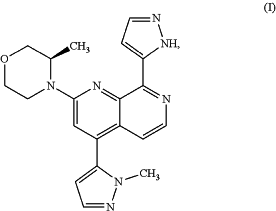
1. A method for preparing a compound of formula (I)
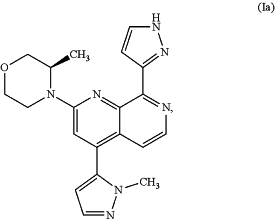
 or its tautomer of formula (Ia)
 or a mixture thereof,
said method comprising the successive steps of:
(a) reacting an intermediate compound of formula (IXa)
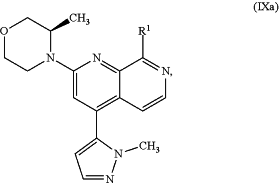 wherein
R1 is a chlorine, bromine or iodine atom or is a group selected from [(trifluoromethyl)sulfonyl]oxy, [(nonafluorobutyl)sulfonyl]oxy, (methylsulfonyl)oxy, (p-toluenesulfonyl)oxy, (phenylsulfonyl)oxy, [(4-bromophenyl)sulfonyl]oxy, [(4-nitrophenyl)sulfonyl]oxy, [(2-nitrophenyl)sulfonyl]oxy, [(4-isopropylphenyl)sulfonyl]oxy, [(2,4,6-triisopropylphenyl)sulfonyl]oxy, [(2,4,6-trimethylphenyl)sulfonyl]oxy, [(4-tert-butylphenyl)sulfonyl]oxy and [(4-methoxyphenyl)sulfonyl]oxy;
with a compound of formula (IIIa) or (IIIb)
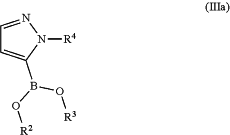 or
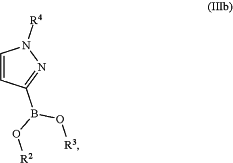 or a mixture thereof,
wherein
R2 and R3 are independently a hydrogen atom or a C1-C6-alkyl group;
or
R2 and R3 together represent a —CH2—CH2— group or a —CH2—CH2—CH2— group, wherein said —CH2—CH2— group or —CH2—CH2—CH2— group is optionally substituted one, two, three or four times with a group selected from methyl or ethyl;
or
R2 and R3 together represent a group
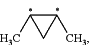 wherein “*” represents the point of attachment to the rest of the molecule;
and
R4 is a group selected from the group consisting of tetrahydro-2H-pyran-2-yl, 1-methyl-1-methoxyethyl, 1-methyl-1-phenoxyethyl, and 1-methyl-1-benzyloxyethyl;
to give an intermediate compound of formula (VIIa) or (VIIb)
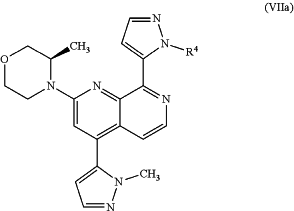 or
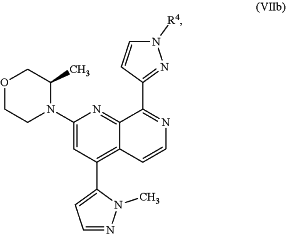 or a mixture thereof; and
(b) removing the group R4 from the intermediate compound of formula (VIIa) or (VIIb), thus providing a compound of formula (I), or its tautomer of formula (Ia), or a mixture thereof.
|