| CPC C07D 405/12 (2013.01) | 22 Claims |
|
1. A compound of formula (I-A-2):
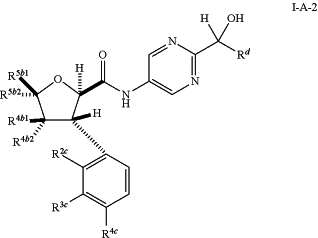 or a pharmaceutically acceptable salt thereof, wherein:
Rd is (CH2)m(CHRe)n(CH2)pH;
m, n, and p are each independently 0 or 1;
Re is H, OH, halo, C1-C6 alkoxy, or C1-C6 haloalkoxy;
R4b1 and R4b2 are each independently H, C1-C6 alkyl, C3-C6 cycloalkyl, or C1-C6 haloalkyl;
R5b1 and R5b2 are each independently H, C1-C6 alkyl, C3-C6 cycloalkyl, or C1-C6 haloalkyl;
R2c is H, OH, halo, C1-C6 alkyl, C2-C6 alkenyl, C1-C6 haloalkyl, C1-C6 alkoxy, C1-C6 haloalkoxy, or -L1-L2-(C3-C6 cycloalkyl), wherein said cycloalkyl is optionally substituted with 1-2 halo;
L1 is a bond or O;
L2 is a bond or C1-C6 alkylene;
R3c is H, halo, C1-C6 alkyl, or C1-C6 haloalkyl; or
R2c and R3c, together with the carbon atoms to which they are attached, form a ring of formula:
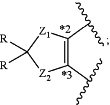 Z1 and Z2 are each independently O or CH2;
each R is independently H or halo; and
R4c is H, halo, C1-C6 alkyl, C1-C6 haloalkyl, C1-C6 alkoxy, or C1-C6 haloalkoxy.
|