| CPC A61F 2/3609 (2013.01) [A61F 2/30907 (2013.01); A61F 2/34 (2013.01); A61F 2/36 (2013.01); A61F 2/38 (2013.01); A61F 2/40 (2013.01); A61F 2/4202 (2013.01); A61F 2002/30387 (2013.01); A61F 2002/30405 (2013.01); A61F 2002/30507 (2013.01); A61F 2002/30517 (2013.01); A61F 2002/3055 (2013.01); A61F 2002/30593 (2013.01); A61F 2002/30672 (2013.01); A61F 2002/30677 (2013.01); A61F 2002/3068 (2013.01); A61F 2002/30784 (2013.01); A61F 2002/30825 (2013.01); A61F 2002/30827 (2013.01); A61F 2002/30878 (2013.01); A61F 2002/30919 (2013.01); A61F 2002/3401 (2013.01); A61F 2002/3613 (2013.01); A61F 2002/3621 (2013.01); A61F 2002/3647 (2013.01); A61F 2002/365 (2013.01); A61F 2002/368 (2013.01); A61F 2002/3694 (2013.01); A61M 2205/04 (2013.01)] | 26 Claims |
|
1. A therapeutic fluid delivery system, said system comprising:
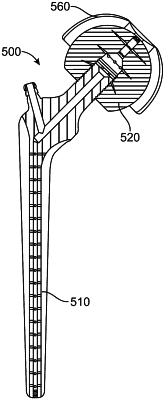
a femoral head comprising:
a surface configured to be disposed in an acetabulum of a patient;
a first threaded portion; and
a first plurality of outlets in the surface of the femoral head;
a femoral stem comprising:
a proximal portion comprising a second threaded portion;
a distal portion configured to be disposed in a femoral medullary canal of said patient;
an inlet; and
a second plurality of outlets in the distal portion of the femoral stem, wherein each outlet of the second plurality of outlets is fluidically coupled to the inlet; and
one or more set screws,
wherein the second threaded portion of the proximal portion of the femoral stem is configured to adjustably threadably engage with the first threaded portion of the femoral head in order to both couple the femoral head to the femoral stem and permit adjustment of a distance between the femoral head and the distal portion of the femoral stem, wherein the one or more set screws releasably lock the femoral head and the femoral stem together to maintain the distance in order to form an assembled system, and
further wherein, when a therapeutic fluid is introduced into the assembled system through the inlet, the therapeutic fluid exits both the second plurality of outlets and the first plurality of outlets.
|