| CPC A01N 53/00 (2013.01) [A01N 37/22 (2013.01); A01N 37/34 (2013.01); A01N 41/10 (2013.01); A01N 43/40 (2013.01); A01N 43/60 (2013.01); A01N 43/653 (2013.01); A01N 43/707 (2013.01); A01N 43/82 (2013.01); A01N 43/90 (2013.01); C07C 233/63 (2013.01); C07C 233/65 (2013.01); C07C 237/22 (2013.01); C07C 237/42 (2013.01); C07C 255/29 (2013.01); C07C 255/46 (2013.01); C07C 255/57 (2013.01); C07C 255/60 (2013.01); C07C 259/10 (2013.01); C07C 271/28 (2013.01); C07C 271/66 (2013.01); C07C 311/08 (2013.01); C07C 311/46 (2013.01); C07C 317/14 (2013.01); C07C 317/28 (2013.01); C07C 317/40 (2013.01); C07C 317/50 (2013.01); C07C 323/41 (2013.01); C07C 323/42 (2013.01); C07C 323/59 (2013.01); C07C 331/12 (2013.01); C07C 381/10 (2013.01); C07D 205/04 (2013.01); C07D 207/10 (2013.01); C07D 207/273 (2013.01); C07D 207/452 (2013.01); C07D 209/49 (2013.01); C07D 213/56 (2013.01); C07D 213/75 (2013.01); C07D 213/81 (2013.01); C07D 213/84 (2013.01); C07D 213/89 (2013.01); C07D 215/227 (2013.01); C07D 215/38 (2013.01); C07D 231/12 (2013.01); C07D 231/56 (2013.01); C07D 233/36 (2013.01); C07D 233/80 (2013.01); C07D 235/30 (2013.01); C07D 241/20 (2013.01); C07D 249/08 (2013.01); C07D 253/07 (2013.01); C07D 261/12 (2013.01); C07D 263/26 (2013.01); C07D 277/30 (2013.01); C07D 277/36 (2013.01); C07D 285/06 (2013.01); C07D 295/32 (2013.01); C07D 305/08 (2013.01); C07D 307/33 (2013.01); C07D 307/52 (2013.01); C07D 309/14 (2013.01); C07D 331/04 (2013.01); C07D 333/36 (2013.01); C07D 333/48 (2013.01); C07D 333/60 (2013.01); C07D 487/04 (2013.01); C07C 2601/02 (2017.05); C07C 2601/04 (2017.05); C07C 2601/08 (2017.05); C07C 2601/14 (2017.05)] | 1 Claim |
|
1. A process comprising
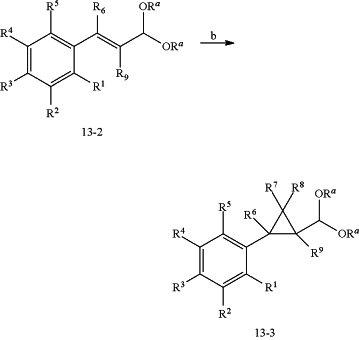 reacting 13-2 with a carbene source comprising a bromoform or a chloroform, in the presence of an inorganic base, and (−)-N-dodecyl-N-methylephedrinium bromide as a phase-transfer catalyst, at a temperature from about ambient temperature up to below the boiling point of the haloform, to form molecule 13-3 wherein
(A) R1 is selected from the group consisting of H, F, or Cl;
(B) R2 is selected from the group consisting of H, F, Cl, Br, (C1-C6)alkyl, and (C1-C6)haloalkyl;
(C) R3 is selected from the group consisting of H, F, Cl, Br, (C1-C6)alkyl, (C1-C6)haloalkyl, and (C1-C6)haloalkoxy;
(D) R4 is selected from the group consisting of H, F, Cl, Br, (C1-C6)alkyl, and (C1-C6)haloalkyl;
(E) R5 is selected from the group consisting of H, F, and Cl;
(F) R6 is H;
(G) R7 is selected from the group consisting of Cl and Br;
(H) R8 is selected from the group consisting of Cl and Br;
(I) R9 is H;
and Ra is a (C1-C6)alkyl.
|