| CPC C07D 519/00 (2013.01) [A61P 7/02 (2018.01); C07D 515/14 (2013.01)] | 19 Claims |
|
1. A compound of Formula (I):
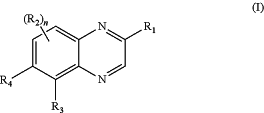 or a salt thereof, wherein
R1 is F, Cl, —OH, C1-4 alkyl, C1-4 fluoroalkyl, C2-4 alkenyl, C2-4 alkynyl, C3-7 cycloalkyl, C3-7 fluorocycloalkyl, C1-4 alkoxy, C1-4 fluoroalkoxy, C2-4 hydroxyalkoxy, C3-6 cycloalkoxy, (C1-3 alkoxy)-(C1-3 alkylene), (C1-3 alkoxy)-(C1-3 fluoroalkylene), (C1-3 deuteroalkoxy)-(C1-3 deuteroalkylene), (C1-3 fluoroalkoxy)-(C1-3 alkylene), (C1-3 fluoroalkoxy)-(C1-3 fluoroalkylene), —(CH2)1-3O(phenyl), —(CH2)13NRaRa, —C(O)O(C1-6 alkyl), —C(O)NRaRa, —C(O)NRbRb, —NH2, —NH(C1-6 alkyl), —N(C1-6 alkyl)2, azetidinyl, pyrrolidinyl, furanyl, pyranyl, piperidinyl, morpholinyl, piperazinyl, —S(O)2(C1-3 alkyl), —S(O)2NRaRa, C1-3 alkylthio, or C1-3 fluoroalkylthio;
R2, at each occurrence, is independently H, F, Cl, Br, —OH, —CN, C1-4 alkyl, C1-4 fluoroalkyl, C1-4 hydroxyalkyl, C1-3 aminoalkyl, C2-4 alkenyl, C2-4 alkynyl, C3-7 cycloalkyl, C3-7 fluorocycloalkyl, C1-6 alkoxy, C1-3 fluoroalkoxy, C1-3 alkylthio, C1-3 fluoroalkylthio, (C1-3 alkoxy)-(C1-3 alkylene), (C1-3 fluoroalkoxy)-(C1-3 alkylene), —C(O)NH2, —C(O)NH(C1-6 alkyl), —C(O)N(C1-6 alkyl)2, —C(O)NRbRb, —CH(OH)(C3-6 cycloalkyl), —CH(OH)(phenyl), CH(OH)(pyridyl), —S(O)2(C1-3 alkyl), —S(O)2NRaRa, or a cyclic group selected from phenyl, 5- to 6-membered heteroaryl, and 5- to 7-membered heterocycle, wherein said cyclic group is substituted with zero to 5 substituents independently selected from F, Cl, hydroxy, C1-3 alkyl, C1-3 alkoxy, cyclopropyl, and —CN;
R3 is:
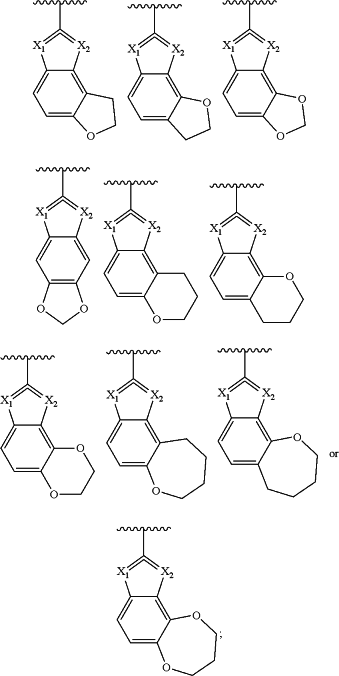 (i) X1 is N and X2 is S, O, or NH;
(ii) X1 is O and X2 is CH or N;
(iii) X1 is NH and X2 is CH; or
(iv) X1 is CH and X2 is S or NH;
and the dashed lines represent the variable position of a double bond to maintain aromaticity, each R3 is substituted with R3a and zero to 3 R3b;
R3a is
(i) H, C1-6 hydroxyalkyl, C1-6 hydroxyfluoroalkyl, —C(O)O(C1-6 alkyl), —CRaRaNHC(O)(C1-6 alkyl), —CRaRaNHC(O)(C1-6 fluoroalkyl), —CRaRaNHC(O)O(C1-6 alkyl), —CRaRaNHC(O)O(CH2)1-3(C1-3 alkoxy), —CRaRaNHC(O)O(C1-4 fluoroalkyl), —CRaRaNHS(O)2(C1-3 alkyl), CRaRaNHS(O)2(C1-3 fluoroalkyl), —CRaRaOP(O)(OH)2, —CRaRaNHC(O)Rx, —CRaRaNHC(O)ORx, —CRaRaNHC(O)CH2Rx, —CRaRaNHC(O)OCH2Rx, —CRaRaOC(O)NHRx, —CRaRaNHC(O)NHRx, —CRaRaORx, or —CRaRaOC(O)Rx;
(ii) —CH(OH)CRhRiRj wherein Rh and Ri are independently H, F, C1-4 alkyl, C1-4 fluoroalkyl, C1-3 alkoxy, or C1-3 fluoroalkoxy, or taken together with the carbon atom to which they are attached, form C3 s cycloalkyl or 4- to 7-membered heterocyclyl ring; and Rj is H, C1-6 alkyl, C1-5 fluoroalkyl, (C1-3 alkoxy)-(C1-3 alkyl), C3 s cycloalkyl, C3 s heterocyclyl, aryl, or heteroaryl;
Rx is C3 6 cycloalkyl, phenyl, pyridinyl, pyridazinyl, pyrimidinyl, pyrazinyl, benzo[d]oxazolyl, benzo[d]thiazolyl, pyrrolopyridinyl, tetrahydroisoquinolinyl, tetrahydrofuranyl, tetrahydropyranyl, morpholinyl, imidazopyridinyl, or oxo-dihydrobenzo[d]oxazolyl, each substituted with zero to two substituents independently selected from F, Cl, Br, —CN, —OH, —CH3, —CF3, C1-3 alkoxy, C1-3fluoroalkyl, C1-6 hydroxyalkyl, C1-6 hydroxyalkoxy, C1-6 hydroxy-fluoroalkoxy, —NRaRa, —C(O)NRaRa, —C(O)NH(C1-6 alkyl), —C(O)N(C1-6 alkyl)2, —C(O)NRbRb, —C(O)NRa(C1-6hydroxyalkyl), —C(O)O(C1-6 alkyl), —C(O)(morpholinyl), —S(O)2NRaRa, —CH(OH)CH20H, —CH═CH2, —NHC(O)CH3, —OCH2CH2N(CH3)2, —OCH2CH20H, —OCH2CH(Me)OH, isoxazolyl, phenoxy, phenyl, pyrrolidinyl, thiophenyl, and methyl triazolyl;
R3b, at each occurrence, is independently H, F, Cl, Br, —CN, C1-3 alkyl, C1-3 fluoroalkyl, C1-3 hydroxyalkyl, —OCHF2, C3-6 cycloalkyl, C3-6 fluorocycloalkyl, C2-4 alkenyl, C2-4 alkynyl, C1-3 alkoxy, C1-3 alkylthio, or C1-3 fluoroalkoxy;
R4 is H, F, Cl, or —CH3;
Ra, at each occurrence, is independently H, C1-4alkyl, or C1-4fluoroalkyl;
two Rb along with the nitrogen atom to which they are attached form a 4- to 7-membered heterocyclo ring having 1 to 2 nitrogen atoms and 0-1 oxygen or sulfur atoms; and
n is zero, 1, or 2.
|