| CPC C07D 401/04 (2013.01) [A61K 31/4545 (2013.01); A61K 31/496 (2013.01); A61K 31/497 (2013.01); A61P 3/10 (2018.01); C07D 211/22 (2013.01); C07D 213/74 (2013.01); C07D 235/14 (2013.01); C07D 239/47 (2013.01); C07D 295/096 (2013.01); C07D 401/12 (2013.01); C07D 401/14 (2013.01); C07D 403/06 (2013.01); C07D 405/14 (2013.01); C07D 413/06 (2013.01); C07D 413/14 (2013.01); C07D 471/04 (2013.01)] | 15 Claims |
|
1. A method for treating a metabolic disease, the method comprising administering to a subject in need a compound represented by following Chemical Formula 1, an optical isomer of the compound, or a pharmaceutically acceptable salt of the compound or the optical isomer:
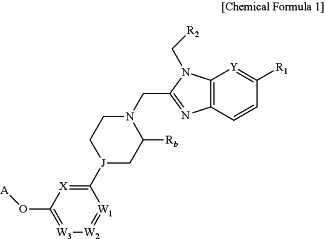 wherein:
R1 is —C(═O)Ra, where Ra is —OH;
Y is —CH— or —N—;
R2 is unsubstituted
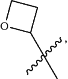 halogen substituted
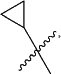 or unsubstituted
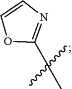 Rb is hydrogen or —(C1-C4 alkyl);
J is —N—;
X is —CRc— or —N—, where Rc is —H;
W1 is —CRd—, where Rd is —H;
W2 is —CRe— or —N—, where Re is —H;
W3 is —CRf—, where Rf is —H; and
A is
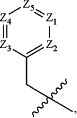 wherein Z1 is —CRg—, where Rg is —H, halogen or —CN;
Z2 is —CRh—, where Rh is —H, halogen or —CN;
Z3 is —N—;
Z4 is —CRj—, where Rj is —H, halogen or —CN; and
Z5 is —CRk—, where Rk is —H, halogen or —CN,
wherein the metabolic diseases are any one selected from the group consisting of diabetes, idiopathic T1D, latent autoimmune diabetes in adults (LADA), early onset T2DM (EOD), younger onset atypical diabetes (YOAD), maturity onset diabetes in young (MODY), malnutrition-related diabetes, gestational diabetes, hyperglycemia, insulin resistance, liver insulin resistance, impaired glucose tolerance, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, visceral fat accumulation, sleep apnea, obesity, eating disorders, dyslipidemia, hyperinsulinemia, non-alcoholic fatty liver disease (NAFLD), atherosclerosis, hypertension, congestive heart failure, myocardial infarction, stroke, hemorrhagic stroke, ischemic stroke, traumatic brain injury, pulmonary hypertension, restenosis after angioplasty, intermittent claudication, metabolic acidosis, ketosis, arthritis, osteoporosis, Parkinson's disease, left ventricular hypertrophy, peripheral arterial disease, loss of vision, cataracts, glomerulosclerosis, chronic renal failure, metabolic syndrome, X syndrome, premenstrual syndrome, angina, thrombosis, transient ischemic attack, vascular restenosis, symptoms of impaired fasting blood sugar, hyperuricemia, gout, erectile dysfunction, psoriasis, foot ulcers, ulcerative colitis, hyper-apo B lipoproteinemia, Alzheimer's disease, schizophrenia, cognitive impairment, inflammatory bowel disease, short bowel syndrome, Crohn's disease, colitis, irritable bowel syndrome and polycystic ovary syndrome.
|