| CPC C07D 275/06 (2013.01) [C07D 239/52 (2013.01); C07D 239/69 (2013.01)] | 17 Claims |
|
1. In a process of making mesosulfuron-methyl which comprises preparing a 1,1,3-trioxo-1,2-benzothiazole-6-carboxamide of formula (I) or a salt thereof
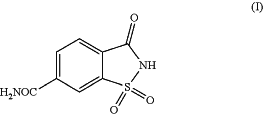 and providing reaction conditions for preparation of mesosulfuron-methyl as follows:
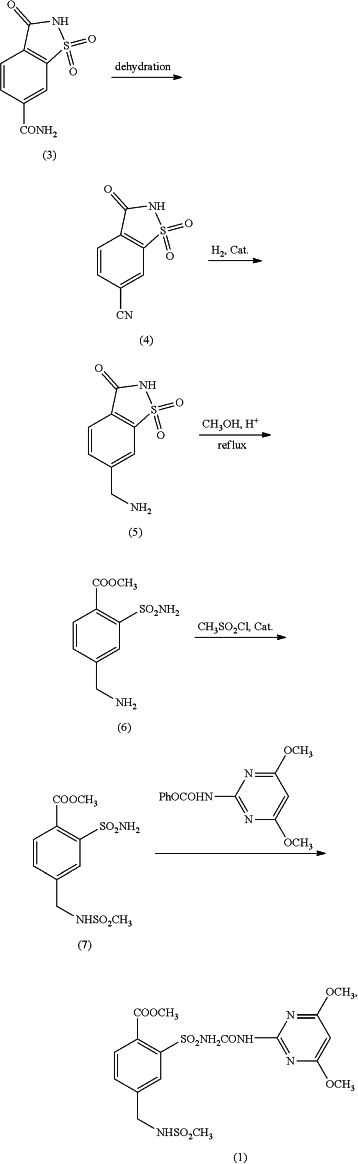 the improvement comprising preparing the 1,1,3-trioxo-1,2-benzothiazole-6-carboxamide of formula (I) or a salt thereof by:
(a) oxidizing 2,5-dimethyl-benzenesulfonamide of formula (II) or a salt thereof;
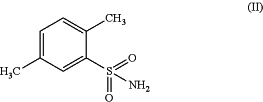 in the presence of an oxidizing agent to obtain 2-sulfamoyl-terephthalic acid of formula (III) or a salt thereof;
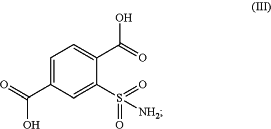 (b) converting the formed 2-sulfamoyl-terephthalic acid of formula (III) to obtain 2-sulfamoyl-terephthalic acid derivative of formula (IV) or a salt thereof;
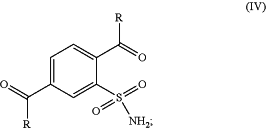 and
(c) reacting the resulting 2-sulfamoyl-terephthalic acid derivative of formula (IV) with ammonia (NH3) or ammonium containing-salt;
wherein R represents OR′ or Cl and R′ represents a branched or non-branched C1-C12 alkyl.
|