|
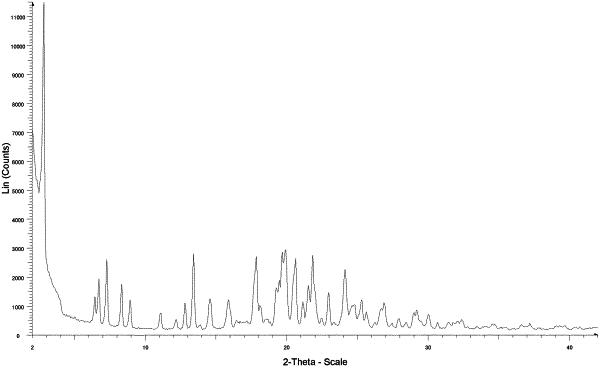
1. A method for treating a primary mitochondrial myopathy (PMM) in a human comprising orally administering to the human with PMM a solid form pharmaceutical composition comprising about 100 mg of crystalline sodium (E)-2-(4-((3-(4-fluorophenyl)-3-(4-(3-morpholinoprop-1-yn-1-yl)phenyl)allyl)oxy)-2-methylphenoxy)acetate (Compound II), wherein the human with PMM has at least one mutation or deletion in at least one mitochondrial DNA (mtDNA) gene; at least one mitochondrial DNA (mtDNA) defect; at least one mutation or deletion in at least one nuclear DNA (nDNA) gene involved in mitochondrial function; or a combination thereof; and wherein crystalline Compound II is characterized as having an XRPD pattern with peaks at 2.8±0.2° 2-Theta, 7.2±0.2° 2-Theta, 13.4±0.2° 2-Theta, 17.8±0.2° 2-Theta, 19.7±0.2° 2-Theta, 19.9±0.2° 2-Theta, and 20.6±0.2° 2-Theta as measured using Cu Kα radiation (crystalline Form 1).
|