| CPC A61K 31/519 (2013.01) [A61K 31/5377 (2013.01); A61K 31/541 (2013.01); A61K 31/551 (2013.01); A61K 45/06 (2013.01); C07D 487/04 (2013.01)] | 22 Claims |
|
1. A method of making a compound of Formula (I), or a pharmaceutically acceptable salt thereof:
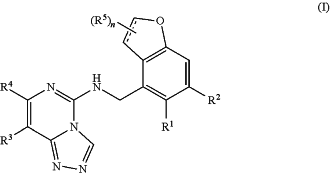 wherein:
 is a single bond or a double bond; is a single bond or a double bond;R1 and R2 are independently H or halogen;
R3 is independently selected from: halogen, phenyl, and a 5- to 6-membered heteroaryl comprising carbon atoms and 1-4 heteroatoms selected from N, NRa, O, and S(O)p; wherein said phenyl and heteroaryl are substituted with 0-3 R3A;
each R3A is independently selected from: halogen, CN, —(O)m—(C1-C6 alkyl substituted with 0-1 R3B), C1-C6 haloalkyl, C1-C6 haloalkoxy, R3C, —OR3C, —C(═O)R3D, NR3ER3F, —C(═O)NR3ER3F, —NHC(═O)R3D, —S(═O)2R3D, —S(═O)2NR3ER3F,
—NHS(═O)2(C1-C4 alkyl), and —CR3CR3ER3G;
R3B is independently selected from: OH, NReRf, C1-C4 alkoxy, —C(═O)NReRf, —S(═O)2(C1-C4 alkyl), —NHC(═O)(C1-C4 alkyl), and a 5- to 6-membered heterocycloalkyl comprising carbon atoms and 1-2 heteroatoms selected from N, NRa, O, and S(O)p; wherein said heterocycloalkyl is substituted with 0-2 Re;
each R3C is independently selected from: C3-C6 cycloalkyl, phenyl, and a 4- to 7-membered heterocycle comprising carbon atoms and 1-4 heteroatoms selected from N, NRa, O, and S(O)p; wherein each moiety is substituted with 0-2 Rc;
each R3D is independently selected from: C1-C4 alkyl and R3C;
R3E and R3G are, at each occurrence, independently selected from: H and C1-C4 alkyl;
each R3F is independently selected from: H and C1-C4 alkyl substituted with 0-1 Rd;
R4 is independently selected from: H, halogen and C1-C4 alkyl;
R5 is independently selected from OH and C1-C4 alkyl;
each Ra is independently selected from: H, →O, C1-C4 alkyl substituted with 0-1 Rb, —C(═O)H, —C(═O)(C1-C4 alkyl), —CO2(C1-C4 alkyl), C3-C6 cycloalkyl, and benzyl;
Rb is independently selected from: halogen, OH and C1-C4 alkoxy;
each Rc is independently selected from: ═O, halogen, OH, C1-C4 alkyl, C1-C4 haloalkyl, C1-C4 alkoxy, and C1-C4 haloalkoxy;
Rd is independently selected from: OH and NReRf;
Re and Rf are, at each occurrence, independently selected from: H and C1-C4 alkyl;
each p is independently selected from 0, 1 and 2; and
m and n are, at each occurrence, independently selected from 0 and 1;
wherein the method comprises the following steps:
i) treating pyrimidine (1) with hydrazine to produce a 4-hydrazinyl pyrimidine (2)
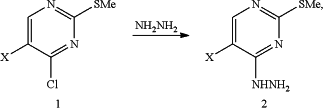 where X is Br or F;
ii) converting 4-hydrazinyl pyrimidine (2) with trimethyl orthoformate to triazole product (3)
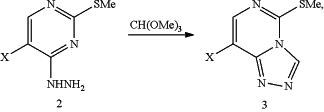 where X is Br or F;
iii) conducting a substitution reaction of triazole product (3) with a compound (A) to produce compound (4)
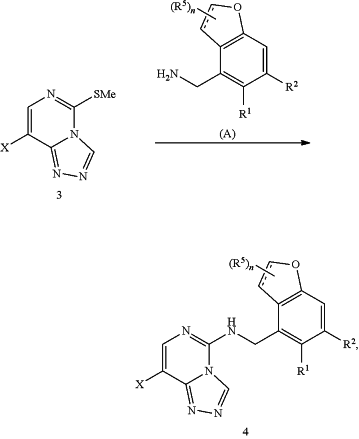 where X is Br or F;
iv) conducting a coupling reaction of compound (4) with reagent R3—B, in the presence of a palladium catalyst, to produce the compound of Formula (I)
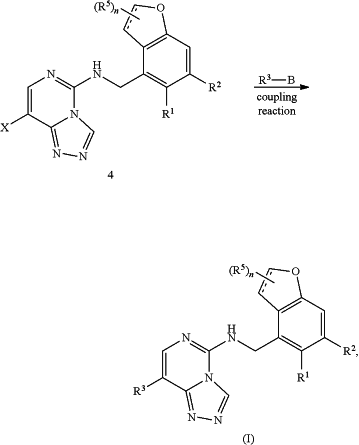 where:
X is Br or F;
R3 is as defined above, and
B is
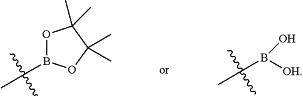 |