| CPC A61H 15/0092 (2013.01) [A61H 15/00 (2013.01); A63B 21/0004 (2013.01); A63B 21/4033 (2015.10); A63B 21/4049 (2015.10); A63B 23/02 (2013.01); A63B 26/003 (2013.01); B29C 44/02 (2013.01); B29C 44/445 (2013.01); B29C 45/00 (2013.01); B29C 48/001 (2019.02); B29C 48/022 (2019.02); B29C 48/03 (2019.02); B29C 48/06 (2019.02); B29C 48/12 (2019.02); A61H 2015/0014 (2013.01); A61H 2201/169 (2013.01); A61H 2201/1695 (2013.01); B29C 53/40 (2013.01); B29C 65/48 (2013.01); B29C 66/128 (2013.01); B29C 66/14 (2013.01); B29C 66/4322 (2013.01); B29C 66/45 (2013.01); B29C 66/71 (2013.01); B29C 66/727 (2013.01); B29D 23/001 (2013.01); B29K 2023/06 (2013.01); B29K 2105/046 (2013.01); B29K 2105/048 (2013.01); B29K 2105/24 (2013.01); B29L 2023/003 (2013.01); B29L 2031/463 (2013.01); B29L 2031/52 (2013.01)] |
| AS A RESULT OF REEXAMINATION, IT HAS BEEN DETERMINED THAT: |
| The patentability of claims 1-16 is confirmed. |
| New claims 17-25 are added and determined to be patentable. |
|
[ 17. The device of claim 1, wherein said soft tissue includes at least one of: muscle, tendon, ligament or fascia.]
|
|
[ 18. The device of claim 1, wherein said soft tissue is fascia.]
|
|
[ 19. The device of claim 1, wherein said soft tissue is muscle.]
|
|
[ 20. The device of claim 1, wherein said soft tissue is muscle and fascia.]
|
|
[ 21. The device of claim 1, wherein said soft tissue is tissue below subcutaneous fat.]
|
|
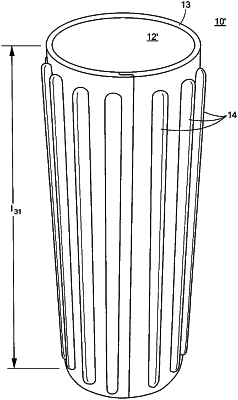
[ 22. A free standing therapeutic, fitness, and sports enhancement device comprising:
a free standing entirely cylindrical shaped core having a first end and a second end, made of closed cell foam, rubber, or plastic and having a diameter of about 3 inches to about 15 inches; and
an overlay completely surrounding the core from about the first end to about the second end, the overlay made of closed cell foam, rubber, or plastic and including a plurality of solid projections having a predetermined shape configured to extend into soft tissue of a user to enhance mobilization of soft tissue and optimize core strength and balance training, wherein the plurality of solid projections extend between about ⅛ of an inch to about 3 inches from the overlay.]
|
|
[ 23. A free standing therapeutic, fitness, and sports enhancement device comprising:
a free standing entirely cylindrical shaped core having a first end and a second end, made of closed cell foam, rubber, or plastic and having a diameter of about 3 inches to about 15 inches; and
an overlay completely surrounding the core from about the first end to about the second end, the overlay made of closed cell foam, rubber, or plastic and including a plurality of solid projections having a predetermined shape configured to extend into soft tissue of a user to enhance mobilization of soft tissue and optimize core strength and balance training, wherein the device is not connected to an external apparatus.]
|
|
[ 24. A free standing therapeutic, fitness, and sports enhancement device comprising:
a free standing entirely cylindrical shaped core having a first end and a second end, made of closed cell foam, rubber, or plastic and having a diameter of about 3 inches to about 15 inches; and
an overlay completely surrounding the core from about the first end to about the second end, the overlay made of closed cell foam, rubber, or plastic and including a plurality of solid projections having a predetermined shape configured to extend into soft tissue of a user to enhance mobilization of soft tissue and optimize core strength and balance training, wherein the plurality of solid projections are integrally molded with the overlay.]
|
|
[ 25. A free standing therapeutic, fitness, and sports enhancement device comprising:
a free standing entirely cylindrical shaped core having a first end and a second end, made of closed cell foam, rubber, or plastic and having a diameter of about 3 inches to about 15 inches; and
an overlay completely surrounding the core from about the first end to about the second end, the overlay made of closed cell foam, rubber, or plastic and including a plurality of solid projections having a predetermined shape configured to extend into soft tissue of a user to enhance mobilization of soft tissue and optimize core strength and balance training, wherein the plurality of solid projections comprise material selected from the group consisting of: closed cell foam; rubber with closed cell foam; and plastic with closed cell foam.]
|