| CPC C07D 471/04 (2013.01) [A61K 31/519 (2013.01); A61K 31/5377 (2013.01); C07D 413/14 (2013.01); C07D 519/00 (2013.01)] | 23 Claims |
|
1. A compound of Formula I
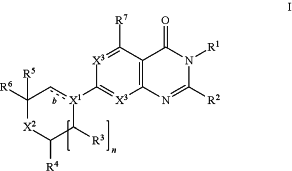 or a tautomer thereof, or a pharmaceutically acceptable salt of said compound or said tautomer, wherein
X1 is (1) CH or N and b is a single bond; or (2) C and b is a double bond;
X2 is CH2, CHF, CF2, O, or NH;
wherein, optionally, R5 is absent and the X2CR6 group forms a 5- or 6-membered heteroaryl, wherein the 5-membered heteroaryl contains only one ring atom selected from N, O, and S and optionally only one further N ring atom and wherein the 6 membered heteroaryl contains only one or only two N ring atoms, and wherein said 5- or 6-membered heteroaryl is optionally substituted with halogen, C1-3alkyl, or C1-3alkoxy;
X3 at each occurrence independently is CH or N;
R1 is H, C1-6alkyl, C1-6haloalkyl, or C3-6cycloalkyl;
R2 is H, C1-3alkyl, C1-3haloalkyl, or C3-6cycloalkyl;
R3 is H or C1-3alkyl;
R4 is H or C1-3alkyl;
R5 is H or C1-3alkyl;
R6 is C2-6alkyl, C1-6haloalkyl, diC1-3alkylamino, —C(═O)O(C1-6alkyl), C3-6cycloalkyl, C3-6heterocycloalkyl, phenyl, 5-membered heteroaryl, or 6-membered heteroaryl; wherein
(1) C3-6cycloalkyl or C3-6heterocycloalkyl is optionally substituted with C═O,
(2) the phenyl, 5-membered heteroaryl, or 6-membered heteroaryl group is optionally substituted with 1 to 3 substituents independently selected from halogen, C1-6alkyl, C1-6haloalkyl, C1-6alkoxy, C1-6haloalkoxy, —(C1-3alkyl)O(C1-3alkyl), —(C1-3alkyl) NH2, —(C1-3alkyl) NH (C1-3alkyl), —(C1-3alkyl)N[(C1-3alkyl) (C1-3alkyl)], —CN, C2-4alkenyl, C3-6cycloalkyl, phenyl, and C3-6heterocycloalkyl; wherein
the C1-6alkyl and C1-6haloalkyl of subsection (2) are optionally substituted with OH; and wherein
the C3-6heterocycloalkyl of subsection (2) is optionally substituted with 1 to 3 substituents selected from halogen, C1-3alkyl, and —C(═O)O(C1-6alkyl);
R7 is C5-6cycloalkyl, C5-8spiroalkyl, C5-8tricycloalkyl, phenyl, or 6-membered heteroaryl; wherein R7 is further optionally substituted with 1 to 4 substituents independently selected from halogen, C1-3alkyl, and C1-3haloalkyl; and
n is 0 or 1; provided that when X1 is N and n is 0, X2 is not NH or O.
|