| CPC B08B 9/093 (2013.01) [A61J 1/065 (2013.01); A61J 1/1468 (2015.05); B08B 17/02 (2013.01); C03C 23/007 (2013.01); C03C 23/0075 (2013.01); C03C 23/0085 (2013.01); G01N 21/9027 (2013.01); G01N 23/2251 (2013.01); G01N 33/386 (2013.01); C11D 2111/18 (2024.01); G01N 2223/652 (2013.01)] | 6 Claims |
|
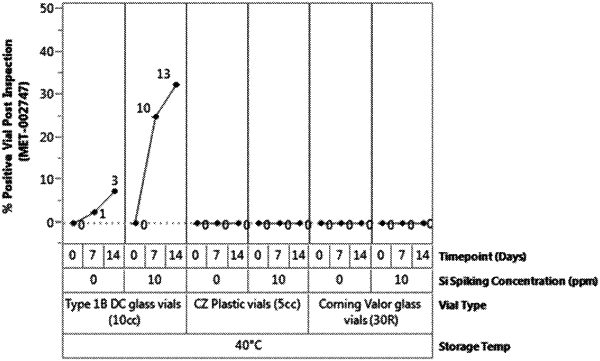
1. A method of screening a glass container for storing a pharmaceutical formulation for susceptibility to lamellar silica formation when filled with the pharmaceutical formulation, comprising:
filling a washed and depyrogenized glass container with a buffer for the pharmaceutical formulation, wherein the glass container comprises ions selected from the group comprising sodium, calcium, hydroxide, silicate, and combinations thereof;
storing the filled glass container at a temperature of 40° C. for at least 14 days;
optically analyzing the buffer after storage for formation of one or more particles in the buffer;
when particle formation is observed, isolating the one or more particles from the buffer for structural and elemental analysis;
analyzing the isolated one or more particles using one or more of optical microscopy, scanning electron microscopy/energy dispersive X-ray spectroscopy (SEM/EDS), Fourier-transform infrared spectroscopy (FTIR), x-ray photoelectron spectroscopy (XPS), differential interference contrast microscopy (DIC), microflow imaging (MFI), and combinations thereof to determine a morphology of the particles and chemical composition; and
analyzing an interior surface of the glass container, once emptied and after isolating the one or more particles, for delamination-type deformation of the interior surface, wherein the presence of particles having a chemical composition comprising silicon, oxygen, and carbon, and an absence of the delamination-type deformation of the interior surface of the glass container indicates the susceptibility to lamellar silica formation.
|