| CPC A61K 9/19 (2013.01) [A61K 38/196 (2013.01); A61K 39/395 (2013.01); A61K 47/02 (2013.01); A61K 47/10 (2013.01); A61K 47/18 (2013.01); A61K 47/183 (2013.01); A61K 47/22 (2013.01); A61K 47/26 (2013.01); A61K 47/40 (2013.01); C07K 14/524 (2013.01); C07K 16/2809 (2013.01); C07K 16/2848 (2013.01); C07K 16/2863 (2013.01); C07K 16/2878 (2013.01); C07K 16/32 (2013.01)] | 22 Claims |
|
1. A process for making a lyophilized pharmaceutical formulation of a therapeutic protein, which comprises:
a. providing a formulation of a bulk amount of the therapeutic protein,
b. measuring the concentration of the therapeutic protein in said bulk formulation,
c. determining a fill weight of the bulk formulation according to the following formula to achieve a target fixed dose of the therapeutic protein in a container,
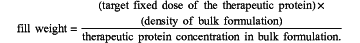 and
d. lyophilizing the formulation in the container.
|