| CPC C07J 31/006 (2013.01) [C07J 9/005 (2013.01)] | 11 Claims |
|
1. A method of treating a CNS-related condition, wherein the CNS-related condition is related to NMDA-modulation and is selected from the group consisting of an adjustment disorder, anxiety disorder, cognitive disorder, dissociative disorder, eating disorder, mood disorder, schizophrenia or other psychotic disorder, sleep disorder, substance-related disorder, personality disorder, autism spectrum disorders, neurodevelopmental disorder, multiple sclerosis, sterol synthesis disorders, pain, encephalopathy secondary to a medical condition, seizure disorder, stroke, traumatic brain injury, movement disorder, vision impairment, hearing loss, or tinnitus, comprising administering to a subject in need thereof an effective amount of a compound of Formula (B):
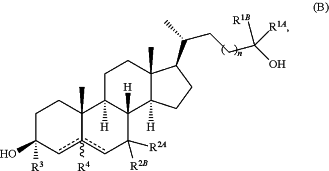 or a pharmaceutically acceptable salt thereof, wherein:
each of R1a and R1B is independently hydrogen, substituted or unsubstituted alkyl, substituted or unsubstituted carbocyclyl, wherein the carbocyclyl may be saturated or partially saturated, substituted or unsubstituted heterocyclyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl, or R1A and R1B, together with the carbon atom to which they are attached form a 3-8 membered ring;
n is 1 or 2;
each of R2A and R2B is independently hydrogen, halo, —ORC, substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl, wherein RC is hydrogen or substituted or unsubstituted alkyl, or R2A and R2B, together with the carbon atom to which they are attached form an oxo group, wherein R2A and R2B are not both simultaneously hydrogen;
R3 is substituted or unsubstituted alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl, or —ORA, wherein RA is substituted or unsubstituted alkyl;
R4 is absent or hydrogen; and
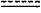 represents a single or double bond, wherein when one of represents a single or double bond, wherein when one of 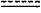 is a double bond, the other is a double bond, the other 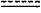 is a single bond; when both of is a single bond; when both of 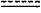 are single bonds, then R4 is hydrogen; and when one of the are single bonds, then R4 is hydrogen; and when one of the 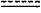 is a double bond, R4 is absent, or is a double bond, R4 is absent, ora pharmaceutical composition comprising said compound of Formula (B) or pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier.
|