| CPC C07C 67/293 (2013.01) [C07B 53/00 (2013.01); C07B 2200/07 (2013.01)] | 33 Claims |
|
1. A process for the synthesis of a non-racemic cyclohexene compound of formula (I)
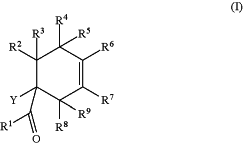 wherein
R1 is selected from C1-C8-alkyl, C3-C12-cycloalkyl, unsubstituted or substituted C6-C20-aryl and unsubstituted or substituted C3-C20-heteroaryl,
R2, R3, R4, R5, R6, R7, R8 and R9 are each independently selected from hydrogen, C1-C8-alkyl, C3-C6-cycloalkyl, unsubstituted or substituted C6-C20-aryl and unsubstituted or substituted C3-C20-heteroaryl, or R5 and R8 together form a bridging moiety selected from —O—, —OH2—, and —CH2—CH2— between the carbon atoms to which they are connected;
Y is OC(O)RA wherein RA is selected from C1-C8-alkyl, C3-C12-cycloalkyl, unsubstituted or substituted C6-C20-aryl, C6-C20-aryl-C1-C4-alkyl, di(C6-C20-aryl)-C1-C4-alkyl, unsubstituted or substituted C3-C20-heteroaryl, C1-C8-alkoxy, C3-C6-cycloalkyloxy, C6-C20-aryloxy, and NRBRB′, where RB and RB, are independently selected from hydrogen, C1-C8-alkyl, and C3-C12-cycloalkyl;
which process comprises reacting a compound of formula (II)
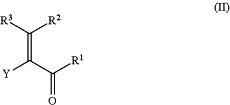 wherein R1, R2, R3 and Y have the same meaning as in formula (I) with a compound of formula III,
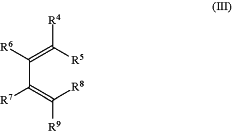 wherein R4, R5, R6, R7, R8 and R9 have the same meaning as in formula (I);
in the presence of a catalyst comprising at least one m-valent metal cation Mm+ wherein the metal M is selected from Scandium (Sc), Yttrium (Y), Lanthanum (La), Cerium (Ce), Praseodymium (Pr), Neodymium (Nd), Promethium (Pm), Samarium (Sm), Europium (Eu), Gadolinium (Gd), Terbium (Tb), Dysprosium (Dy), Holmium (Ho), Erbium (Er), Thulium (Tm), Ytterbium (Yb), Lutetium (Lu), Gallium (Ga) and Indium (In), and m is an integer of 1, 2 or 3, and a chiral ligand of the formula (IV)
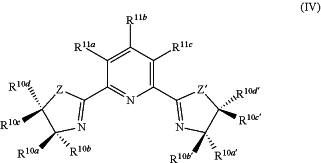 wherein R10a, R10b, R10c, R10d, R10a′, R10b′, R10c′ and R10d′ are each independently selected from hydrogen, C1-C8-alkyl, C3-C6-cycloalkyl, unsubstituted or substituted C6-C20-aryl, C6-C20-aryl-C1-C4-alkyl and unsubstituted or substituted C3-C20-heteroaryl, or two or more of R10a, R10b, R10c and R10d and/or two or more of R10a′, R10b′, R10c′ and R10d′ together form an unsubstituted or substituted ring selected from C3-C6-cycloalkyl, C6-C20-aryl and C3-C20-heteroaryl;
provided that at least one of R10a, R10b, R10c and R10d and at least one of R10a′, R10b′, R10c′ and R10d′ are not hydrogen;
R11a, R11b and R11c are each independently selected from hydrogen, halogen, cyano, C1-C8-alkyl, C1-C4-haloalkyl, C3-C6-cycloalkyl, unsubstituted or substituted C6-C20-aryl, unsubstituted or substituted C3-C20-heteroaryl, C1-C5-alkoxy, C3-C6-cycloalkyloxy, C6-C2O-aryloxy, C(O)—O—C1-C6-alkyl and O—C(O)—C1-C6-alkyl, and
Z and Z′ are the same or different and selected from —O—, —O—CH2—,
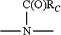 wherein RC is selected from C1-C8-alkyl, C3-C8-cycloalkyl, C1-C8-haloalkyl, and unsubstituted or substituted C6-C13 aryl.
|