| CPC C07C 51/04 (2013.01) [C07C 59/285 (2013.01); C07B 2200/13 (2013.01)] | 20 Claims |
|
1. A method of lowering low-density lipoprotein cholesterol (LDL-C) in a human in need thereof comprising administering to the human a therapeutically effective amount of a pharmaceutical material comprising a crystalline form of the compound of formula (V):
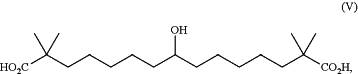 or a pharmaceutically acceptable salt thereof;
wherein the pharmaceutical material comprises the compound of formula (V), or a pharmaceutically acceptable salt thereof, in an amount greater than 99.0% by weight based on the total weight of the pharmaceutical material, and the pharmaceutical material comprises 0.0001% to less than or equal to 0.15% of a compound of formula (VI):
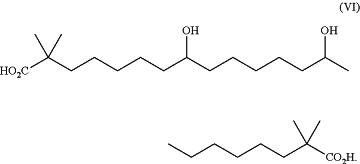 |