| CPC A61K 9/2077 (2013.01) [A61K 9/2009 (2013.01); A61K 9/2013 (2013.01); A61K 9/2027 (2013.01); A61K 9/2031 (2013.01); A61K 9/2054 (2013.01); A61K 31/437 (2013.01); A61K 31/4545 (2013.01); A61K 9/146 (2013.01)] | 10 Claims |
|
1. A tablet comprising:
an extrudate comprising:
a water-soluble polymer matrix;
a dispersing agent; and
a compound of Formula Ia, or a pharmaceutically acceptable salt thereof:
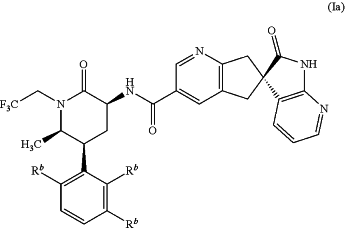 wherein each of Rb is —H,
wherein the compound of Formula Ia is dispersed within the polymer matrix; and
a disintegration system.
|