| CPC A61K 9/1277 (2013.01) [A61K 31/451 (2013.01); B01D 61/146 (2022.08); B01D 61/147 (2013.01); B01D 61/1471 (2022.08); B01F 23/4105 (2022.01); B01F 23/808 (2022.01); B01D 2315/10 (2013.01); B01D 2315/16 (2013.01); B01F 23/4144 (2022.01); B01F 23/4145 (2022.01); B01F 2101/22 (2022.01); B01F 2215/044 (2013.01); B01F 2215/0477 (2013.01); B01F 2215/0481 (2013.01)] | 29 Claims |
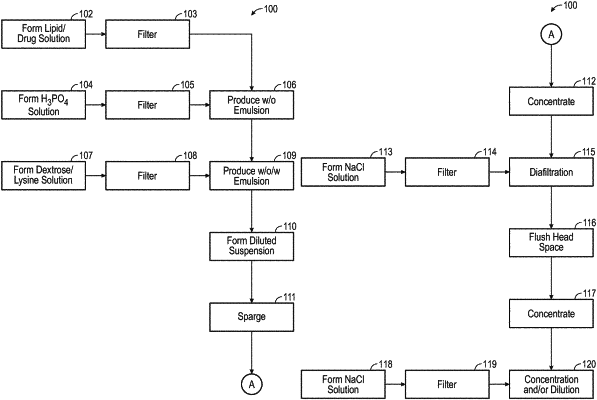
|
1. A composition of bupivacaine encapsulated multivesicular liposomes (MVLs), comprising:
bupivacaine encapsulated MVLs comprising bupivacaine residing inside a plurality of internal aqueous chambers of MVLs separated by lipid membranes, wherein the lipid membranes comprise 1, 2-dierucoylphosphatidylcholine (DEPC), 1, 2-dipalmitoyl-sn-glycero-3-phospho-rac-(1-glycerol) (DPPG), cholesterol, and at least one neutral lipid, the plurality of internal aqueous chambers of the MVLs also comprise lysine; and
an aqueous medium in which the bupivacaine encapsulated MVLs are suspended, wherein the aqueous medium also comprises unencapsulated bupivacaine;
wherein the bupivacaine concentration in the composition is from about 11.3 mg/mL to about 17.0 mg/mL;
wherein an erucic acid concentration in the composition is about 53 μg/mL or less when measured after the composition is stored at 25° C. for three months, and the erucic acid concentration in the composition is about 99 μg/mL or less when measured after the composition is stored at 25° C. for six months; and
wherein the encapsulated lysine concentration in the composition is at least about 0.03 mg/mL.
|