| CPC A61K 31/4545 (2013.01) [A61K 31/444 (2013.01); A61K 31/5386 (2013.01); C07D 471/04 (2013.01); C07D 519/00 (2013.01)] | 37 Claims |
|
1. A method of inhibiting increased HPK1 activity in a subject in need thereof, comprising administering to the subject a pharmaceutical composition comprising of a compound of Formula I,
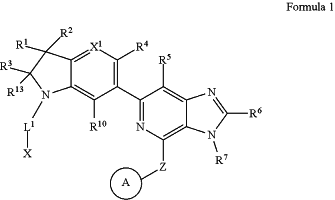 or a pharmaceutically acceptable salt thereof,
wherein:
one of R1 and R2 is H, —CN, —OH, halogen, or C1-6 alkyl, and the other of R1 and R2 is H, halogen, or C1-6 alkyl, wherein each C1-6 alkyl is optionally substituted with 1-3 groups independently selected from —OH and halogen, or
R1 and R2 together with the carbon to which they are attached form a C3-7 monocyclic cycloalkyl or a 4-6 membered monocyclic heterocyclyl having 1 or 2 heteroatoms independently selected from N, O, and S, wherein the C3-7 monocyclic cycloalkyl and the 4-6 membered monocyclic heterocyclyl are each optionally substituted with one R11 and are each optionally substituted with 1-3 groups independently selected from —OH, halogen, oxo, C1-3 alkyl, and C1-3 alkoxy, or
R1 and R2 together form ═O;
R11 is
i) 4-6 membered monocyclic heterocyclyl having 1 or 2 heteroatoms independently selected from N, O, and S, wherein the 4-6 membered monocyclic heterocyclyl is optionally substituted with 1-3 groups independently selected from —CN, —OH, halogen, oxo, C1-3 alkyl, and C1-3 alkoxy,
ii) —S(O)2C1-6 alkyl,
iii) —S(O)2C3-7 monocyclic cycloalkyl,
iv) C1-6 alkyl optionally substituted with 1-3 groups independently selected from —CN, —OH, halogen, C1-3 alkoxy, and C3-7 monocyclic cycloalkyl, or
v) —C(O)R21;
R21 is
i) H,
ii) C3-7 monocyclic or bridged bicyclic cycloalkyl optionally substituted with 1-3 groups independently selected from —CN, —OH, halogen, C1-3 alkyl, and C1-3 alkoxy, wherein the C1-3 alkyl is optionally substituted with 1-3 groups independently selected from —OH, halogen, and C1-3 alkoxy,
iii) 4-6 membered monocyclic heterocyclyl having 1 or 2 heteroatoms independently selected from N, O, and S, wherein the 4-6 membered monocyclic heterocyclyl is optionally substituted with 1-3 groups independently selected from —CN, —OH, halogen, oxo, C1-3 alkyl, and C1-3 alkoxy,
iv) 5-6 membered monocyclic heteroaryl having 1-4 heteroatoms independently selected from N, O, and S, wherein the 5-6 membered monocyclic heteroaryl is optionally substituted with 1-3 groups independently selected from —CN, —OH, halogen, C1-3 alkyl, and C1-3 alkoxy,
v) —NH2,
vi) —NH(C1-6 alkyl), wherein the C1-6 alkyl is optionally substituted with 1-3 groups independently selected from —CN, —OH, halogen, and C1-3 alkoxy,
vii) —N(C1-6 alkyl)2, wherein each C1-6 alkyl can be the same or different and wherein each C1-6 alkyl is optionally substituted with 1-3 groups independently selected from —CN, —OH, halogen, and C1-3 alkoxy,
viii) C1-6 alkoxy optionally substituted with 1-3 groups independently selected from —OH, halogen, C1-3 alkyl, and C3-7 monocyclic cycloalkyl, or
ix) C1-6 alkyl optionally substituted with 1-3 groups independently selected from
a) —CN,
b) —OH,
c) halogen,
d) C1-3 alkoxy,
e) C3-7 monocyclic cycloalkyl optionally substituted with 1-3 groups independently selected from —CN, —OH, halogen, C1-3 alkyl, and C1-3 alkoxy,
f) 4-6 membered monocyclic heterocyclyl having 1 or 2 heteroatoms independently selected from N, O, and S, wherein the 4-6 membered monocyclic heterocyclyl is optionally substituted with 1-3 groups independently selected from —CN, —OH, halogen, oxo, C1-3 alkyl, and C1-3 alkoxy, and
g) —OC(O)C1-6 alkyl optionally substituted with one —OH;
R3 and R13 are each H, or
R3 and R13 together form ═O;
L1 is a cyclobutylene optionally substituted with 1-6 groups independently selected from —OH, halogen, C1-3 alkyl, and C1-3 alkoxy;
X is —NR15R16, wherein R15 and R16 are independently
i) H,
ii) C3-7 monocyclic cycloalkyl optionally substituted with 1-3 groups independently selected from —OH, halogen, C1-3 alkyl, and C1-3 alkoxy,
iii) 4-7 membered monocyclic heterocyclyl having 1 or 2 heteroatoms independently selected from N, O, and S, wherein the 4-7 membered monocyclic heterocyclyl is optionally substituted with 1-3 groups independently selected from —OH, halogen, oxo, C1-3 alkyl, and C1-3 alkoxy,
iv) —C(O)C1-6 alkyl, wherein the C1-6 alkyl is optionally substituted with 1-3 groups independently selected from —CN, —OH, halogen, and C1-3 alkoxy, or
v) C1-6 alkyl optionally substituted with 1-6 groups independently selected from
a) —CN,
b) —OH,
c) halogen,
d) C1-3 alkoxy,
e) C3-7 monocyclic cycloalkyl optionally substituted with 1-3 groups independently selected from —OH, halogen, C1-3 alkyl, and C1-3 alkoxy, and
f) 5-6 membered monocyclic heterocyclyl having 1 or 2 heteroatoms independently selected from N, O, and S, wherein the 5-6 membered monocyclic heterocyclyl is optionally substituted with 1-3 groups independently selected from —OH, halogen, oxo, C1-3 alkyl, and C1-3 alkoxy; or
X is a 4-10 membered monocyclic, fused bicyclic, bridged bicyclic, or spirocyclic heterocyclyl having 1-3 heteroatoms independently selected from N, O, and S, wherein the 4-10 membered monocyclic, fused bicyclic, bridged bicyclic, or spirocyclic heterocyclyl is optionally substituted with 1-5 R18;
each R18 is independently
i) —CN,
ii) a halogen,
iii) —OH,
iv) C1-6 alkoxy optionally substituted with 1-3 groups independently selected from —OH, halogen, C1-3 alkoxy, and C3-7 monocyclic cycloalkyl,
v) C1-6 alkyl optionally substituted with 1-3 groups independently selected from —OH, halogen, C1-3 alkoxy, and C3-7 monocyclic cycloalkyl,
vi) —COOH, or
vii) —C(O)N(R22)2, wherein each R22 is independently H or C1-6 alkyl;
X1 is N or CR17;
R4, R5, R6, R10, and R17 are each independently H, halogen, C1-3 alkyl, or C1-3 alkoxy;
R7 is
i) H,
ii) C1-6 alkyl optionally substituted with 1-3 groups independently selected from —OH, halogen, C1-3 alkoxy, and C3-7 monocyclic cycloalkyl, or
iii) C3-7 monocyclic cycloalkyl optionally substituted with 1-3 groups independently selected from —OH, halogen, C1-3 alkyl, and C1-3 alkoxy;
Z is —O—, —C(R8)2—, or —NR8—;
each R8 is independently H or C1-3 alkyl;
A is a pyridinyl, pyridonyl, quinolinyl, or isoquinolinyl, each of which is optionally substituted with 1-4 R9;
each R9 is independently
i) halogen,
ii) C1-6 alkoxy optionally substituted with 1-3 groups independently selected from —OH, halogen, C1-3 alkyl, and C3-7 monocyclic cycloalkyl,
iii) —NH2,
iv) —NH(C1-6 alkyl), wherein the C1-6 alkyl is optionally substituted with 1-3 groups independently selected from —OH, halogen, and C1-3 alkoxy,
v) —N(C1-6 alkyl)2, wherein each C1-6 alkyl can be the same or different, and wherein each C1-6 alkyl is optionally substituted with 1-3 groups independently selected from —OH, halogen, and C1-3 alkoxy,
vi) —P(O)(C1-6 alkyl)2, wherein each C1-6 alkyl can be the same or different, and wherein each C1-6 alkyl is optionally substituted with 1-3 groups independently selected from —OH, halogen, and C1-3 alkoxy,
vii) —S(O)2C1-6 alkyl,
viii) —S(O)2N(R23)2, wherein each R23 is independently H or C1-6 alkyl,
ix) C1-6 alkyl optionally substituted with 1-3 groups independently selected from
a) —OH,
b) halogen,
c) C1-3 alkoxy,
d) C3-7 monocyclic cycloalkyl,
e) 5-6 membered monocyclic heterocyclyl having 1 or 2 heteroatoms independently selected from N, O, and S, wherein the 5-6 membered monocyclic heterocyclyl is optionally substituted with 1-3 groups independently selected from oxo and C1-3 alkyl, and
f) —NR20C(O)OC1-3 alkyl, wherein R20 is H or C1-3 alkyl,
x) C3-7 monocyclic cycloalkyl optionally substituted with 1-3 groups independently selected from —OH, halogen, C1-3 alkyl, and C1-3 alkoxy,
xi) 5-6 membered monocyclic heteroaryl having 1-4 heteroatoms independently selected from N, O, and S, wherein the 5-6 membered monocyclic heteroaryl is optionally substituted with 1-3 groups independently selected from —OH, halogen, C1-3 alkyl, and C1-3 alkoxy,
xii) 4-6 membered monocyclic heterocyclyl having 1-3 heteroatoms independently selected from N, O, and S, wherein the 4-6 membered monocyclic heterocyclyl is optionally substituted with 1-3 groups independently selected from —OH, halogen, oxo, C1-3 alkyl, and C1-3 alkoxy,
xiii) —COOH,
xiv) —C(O)N(R19)2, or
xv) —C1-3 alkylC(O)N(R19)2; and
each R19 is independently
i) H,
ii) —S(O)2C1-6 alkyl,
iii) C1-6 alkyl optionally substituted with 1-6 groups independently selected from —CN, —OH, halogen, C1-3 alkoxy, and C3-7 monocyclic cycloalkyl,
iv) C3-7 monocyclic cycloalkyl optionally substituted with 1-6 groups independently selected from —CN, —OH, halogen, C1-6 alkyl, and C1-6 alkoxy, wherein the C1-6 alkyl is optionally substituted with 1-3 groups independently selected from —CN, —OH, halogen, and C1-3 alkoxy, or
v) 4-6 membered monocyclic heterocyclyl having 1-3 heteroatoms independently selected from N, O, and S, wherein the 4-6 membered monocyclic heterocyclyl is optionally substituted with 1-6 groups independently selected from —CN, —OH, halogen, oxo, C1-3 alkyl, and C1-3 alkoxy.
|