| CPC A61K 31/437 (2013.01) [A61K 9/0053 (2013.01); A61K 9/4808 (2013.01); A61K 9/4866 (2013.01); A61K 47/10 (2013.01); A61K 47/14 (2013.01); A61K 47/26 (2013.01)] | 21 Claims |
|
1. A formulation for oral administration of relacorilant, (R)-(1-(4-fluorophenyl)-6-((1-methyl-1H-pyrazol-4-yl)sulfonyl)-4,4a,5,6,7,8-hexahydro-1H-pyrazolo[3,4-g]isoquinolin-4a-yl)(4-(trifluoromethyl)pyridin-2-yl)methanone, which has the following structure:
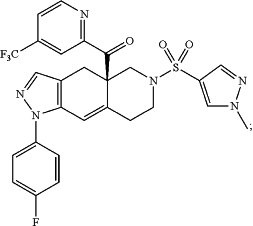 the formulation comprising:
about 18 to 22% relacorilant;
about 56% to 64% surfactant, wherein said surfactant is lauroyl polyoxyl glyceride, and
about 18% to 22% solubilizer, wherein said solubilizer is propylene glycol monocaprylate,
wherein said percentages are weight percent.
|