| CPC A61B 18/1492 (2013.01) [A61B 34/20 (2016.02); A61B 2018/0022 (2013.01); A61B 2018/00541 (2013.01); A61B 2018/00577 (2013.01); A61B 2018/00642 (2013.01); A61B 2018/00791 (2013.01); A61B 2018/00875 (2013.01); A61B 2018/00982 (2013.01); A61B 2018/048 (2013.01); A61B 2018/1425 (2013.01); A61B 2018/1467 (2013.01); A61B 2018/1472 (2013.01); A61B 2034/2051 (2016.02); A61B 2034/2061 (2016.02); A61B 2034/2063 (2016.02); A61B 2218/002 (2013.01); A61B 2218/007 (2013.01)] | 21 Claims |
|
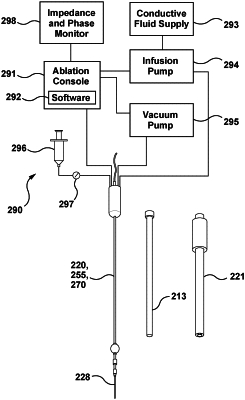
1. An ablation catheter assembly configured to ablate tissue in a lung of a patient, the ablation catheter assembly comprising:
a flexible shaft configured to advance endobronchially into an airway of the lung;
an ablation electrode attached to a distal portion of the flexible shaft and configured to deliver radiofrequency (RF) electrical current to the tissue, wherein the ablation electrode is conductively connectable to an RF electrical energy source external to the patient;
a liquid outlet on the distal portion and configured to be in fluid communication with a source of a conductive liquid;
a first occluder attached to the flexible shaft proximal to the ablation electrode and proximal to the liquid outlet;
a suction opening at the distal portion of the flexible shaft, wherein the suction opening is configured to be in fluid communication with a vacuum;
a second electrode on the flexible shaft between the ablation electrode and the first occluder;
an impedance monitoring circuit configured to measure an impedance between the ablation electrode and the second electrode; and
a controller configured to automatically stop suction through the suction opening in response to a reduction of at least five percent in the impedance between the ablation electrode and the second electrode.
|