| CPC H10K 85/6572 (2023.02) [C07D 209/94 (2013.01); C07D 405/14 (2013.01); C09K 11/06 (2013.01); H10K 85/6574 (2023.02); C09K 2211/1025 (2013.01); H10K 50/11 (2023.02); H10K 50/15 (2023.02); H10K 50/17 (2023.02); H10K 50/18 (2023.02); H10K 2101/10 (2023.02)] | 7 Claims |
|
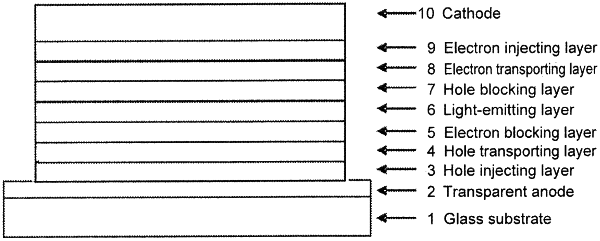
6. An organic electroluminescence device having a light-emitting layer and other layer between a pair of electrodes,
wherein the other layer is a hole injecting layer, a hole transporting layer, or an electron blocking layer,
wherein an indenocarbazole compound is adapted to be used as a constituent material of the light-emitting layer or the other layer,
wherein the indenocarbazole compound has hole transporting properties and is represented by the following general Chemical formula (1)
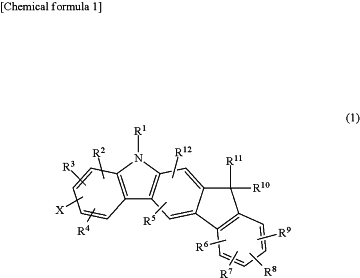 wherein R1 represents an unsubstituted phenyl group, an unsubstituted diphenyl group, or an unsubstituted dibenzofuranyl group;
wherein R2 to R12 may be the same or different and each represents a hydrogen atom, a deuterium atom, a fluorine atom, a chlorine atom, a cyano group, a nitro group, an alkyl group having 1 to 8 carbon atoms, a cycloalkyl group having 5 to 10 carbon atoms, an alkenyl group having 2 to 6 carbon atoms, an alkyloxy group having 1 to 6 carbon atoms, a cycloalkyloxy group having 5 to 10 carbon atoms, an aromatic hydrocarbon group, an aromatic heterocyclic group, an aryloxy group, or disubstituted amino group substituted with an aromatic hydrocarbon group or an aromatic heterocyclic group; R1 to R12 may bond to form a ring via a single bond, a substituted or unsubstituted methylene group, an oxygen atom, or a sulfur atom; and wherein R10 and R11 are not bonded to each other via a single bond, a substituted or unsubstituted methylene group, an oxygen atom, or a sulfur atom; and
wherein X represents an unsubstituted carbazolyl group or an unsubstituted dibenzofuranyl group.
|