| CPC H04L 67/125 (2013.01) [G06F 8/65 (2013.01); G06F 9/452 (2018.02); G16H 40/40 (2018.01); G16H 40/67 (2018.01); H04L 5/14 (2013.01); H04L 41/08 (2013.01); H04L 41/20 (2013.01); H04L 41/22 (2013.01); H04L 43/16 (2013.01); H04L 67/34 (2013.01); H04W 84/12 (2013.01); H02J 7/0048 (2020.01)] | 25 Claims |
|
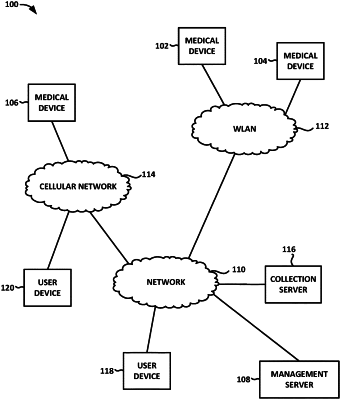
1. A medical device system for use in patient resuscitation and medical device management, the medical device system comprising:
a fleet of medical devices associated with a common administrator and distributed over multiple locations, each medical device comprising:
a first memory,
a treatment component configured to provide treatment to a patient,
a first processor communicably coupled to the first memory and the treatment component, the first processor being configured to store medical device information comprising:
device readiness information that indicates an expiration date associated with the treatment component, and
clinical event information observed by the medical device during a use of the medical device during a clinical event, and
a first communication component communicably coupled to the first processor and configured to transmit the stored medical device information via a network; and
one or more servers communicatively coupled to the fleet of medical devices and one or more user devices located remotely from the one or more servers, the one or more servers comprising:
at least one second communication component configured to receive the medical device information transmitted from the fleet of medical devices by each first communication component,
at least one second memory configured to store the received medical device information, and
at least one second processor configured to provide a report comprising the device readiness information for the fleet of medical devices at a medical device dashboard accessible by the one or more user devices, wherein the report indicates the expiration date associated with at least some of the medical devices in the fleet of medical devices.
|