| CPC C07D 471/10 (2013.01) [A61P 35/02 (2018.01); C07D 519/00 (2013.01)] | 28 Claims |
|
1. A compound of Formula 0,
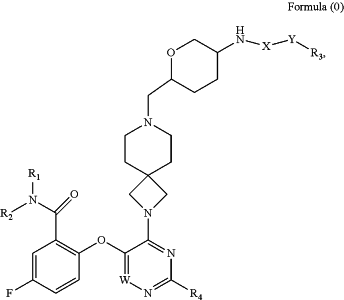 a stereoisomer thereof, or a pharmaceutically acceptable salt thereof, wherein
W is N or CH;
X is C═O, S(═O)(═NR5), or S(═O)2;
Y is NH, O, or a bond;
R1 is C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C1-C6 alkoxy, C3-C12 cycloalkyl, C6-C10 aryl, 5- to 10-membered heteroaryl, or 3- to 12-membered heterocyclyl; wherein the alkyl, alkenyl, alkynyl, alkoxy, cycloalkyl, aryl, heteroaryl, or heterocyclyl is optionally substituted by one or more halo, OH, OBn, oxo, CN, N(RN)2, C1-C6 alkyl, C1-C6 haloalkyl, C3-C6 cycloalkyl, or C1-C6 alkoxy;
R2 is C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C1-C6 alkoxy, C3-C12 cycloalkyl, C6-C10 aryl, 5- to 10-membered heteroaryl, or 3- to 12-membered heterocyclyl; wherein the alkyl, alkenyl, alkynyl, alkoxy, cycloalkyl, aryl, heteroaryl, or heterocyclyl is optionally substituted with one or more halo, OH, OBn, oxo, CN, N(RN)2, C1-C6 alkyl, C1-C6 haloalkyl, C3-C6 cycloalkyl, or C1-C6 alkoxy;
R1 and R2 optionally form a 3- to 12-membered heterocyclyl, wherein the heterocyclyl is optionally substituted with one or more C1-C6 alkyl, halo, OH, CN, or C1-C6 alkoxy;
R3 is H, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C1-C6 alkoxy, C3-C12 cycloalkyl, NH2, NH—C1-C6 alkyl, N—(C1-C6 alkyl)2, C6-C10 aryl, 5- to 10-membered heteroaryl, 3- to 12-membered heterocyclyl, wherein the alkyl, alkenyl, alkynyl, alkoxy, cycloalkyl, aryl, heteroaryl, or heterocyclyl is optionally substituted with one or more halo, OH, OBn, oxo, CN, N(RN)2, C1-C6 alkyl, C1-C6 haloalkyl, C3-C6 cycloalkyl, C1-C6 alkoxy or aryl;
R4 is H, halo, C1-C6 alkyl, C1-C6 alkoxy, C1-C6 haloalkyl, or N(RN)2;
each RN is independently H, C1-C6 alkyl, or C1-C6 haloalkyl; and
each R5 is independently H, C1-C6 alkyl, or C1-C6 haloalkyl.
|