| CPC C07C 5/13 (2013.01) [C07C 1/20 (2013.01); C10L 1/06 (2013.01); C07C 2531/025 (2013.01); C10L 2200/0484 (2013.01); C10L 2270/04 (2013.01)] | 18 Claims |
|
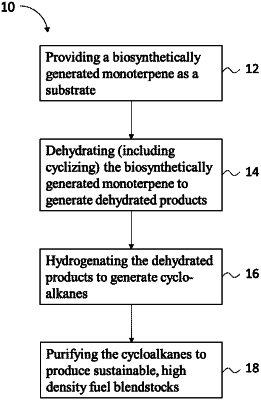
1. A method of converting monoterpenes to cycloalkanes useful as high performance aviation fuel blendstocks, the method comprising:
providing an oxygenated acyclic monoterpene as a substrate;
dehydrating the substrate to generate a dehydrated product in a reaction vessel;
hydrogenating the dehydrated product in the reaction vessel to generate cycloalkanes; and
purifying the cycloalkanes, thereby producing a fuel blendstock, wherein
the oxygenated acyclic monoterpene is obtained from one or more plant extracts or is synthesized from biological sources,
dehydrating the substrate comprises creating a vacuum in the reaction vessel or filling the reaction vessel with an inert gas, and heating the reaction vessel to a temperature of about 0° C. to less than about 50° C. for about 1 hour to about 6 hours,
hydrogenating the dehydrated products comprises creating a hydrogen atmosphere by pressurizing the reaction vessel using hydrogen to a pressure of about 500 pounds per square inch, and performing a hydrogenation reaction at one or more temperatures from about 25° C. to about 150° C. for about 1 hour to about 15 hours, and
dehydrating the substrate and hydrogenating the dehydrated product are performed without the use of a solvent.
|