| CPC C07C 259/06 (2013.01) [A61K 31/185 (2013.01); A61K 31/277 (2013.01); A61K 31/397 (2013.01); A61K 31/4035 (2013.01); A61K 31/407 (2013.01); A61K 31/417 (2013.01); A61K 31/4184 (2013.01); A61K 31/435 (2013.01); A61K 31/438 (2013.01); A61K 31/44 (2013.01); A61K 31/4439 (2013.01); A61K 31/451 (2013.01); A61K 31/495 (2013.01); A61K 31/5377 (2013.01); C07D 207/10 (2013.01); C07D 207/267 (2013.01); C07D 209/08 (2013.01); C07D 209/42 (2013.01); C07D 209/44 (2013.01); C07D 209/46 (2013.01); C07D 211/16 (2013.01); C07D 211/58 (2013.01); C07D 213/56 (2013.01); C07D 213/65 (2013.01); C07D 213/75 (2013.01); C07D 213/81 (2013.01); C07D 215/12 (2013.01); C07D 221/20 (2013.01); C07D 231/14 (2013.01); C07D 231/18 (2013.01); C07D 233/36 (2013.01); C07D 233/38 (2013.01); C07D 233/68 (2013.01); C07D 235/14 (2013.01); C07D 235/30 (2013.01); C07D 239/20 (2013.01); C07D 239/22 (2013.01); C07D 241/08 (2013.01); C07D 241/12 (2013.01); C07D 249/18 (2013.01); C07D 257/04 (2013.01); C07D 261/18 (2013.01); C07D 277/56 (2013.01); C07D 295/155 (2013.01); C07D 295/192 (2013.01); C07D 307/68 (2013.01); C07D 317/68 (2013.01); C07D 333/70 (2013.01); C07D 401/04 (2013.01); C07D 401/12 (2013.01); C07D 401/14 (2013.01); C07D 405/12 (2013.01); C07D 409/04 (2013.01); C07D 409/12 (2013.01); C07D 417/04 (2013.01); C07D 417/12 (2013.01); C07D 471/04 (2013.01); C07D 471/10 (2013.01); C07D 487/04 (2013.01); C07D 487/08 (2013.01); C07D 487/10 (2013.01); C07D 491/10 (2013.01); C07D 493/08 (2013.01); C07D 495/10 (2013.01); C07D 498/04 (2013.01); C07D 513/10 (2013.01); Y02A 50/30 (2018.01)] | 20 Claims |
|
1. A method of treating, inhibiting, or eliminating a disease or disorder associated with inhibition of HDAC8, the method comprising administering to a patient in need thereof a therapeutically effective amount of a compound of formula (I):
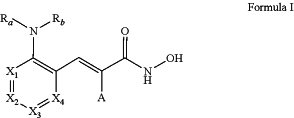 or a pharmaceutically acceptable salt thereof,
wherein:
X1, X2, X3, and X4 are each CH;
Ra is hydrogen;
Rb is —C(O)Rc;
or alternatively, Ra and Rb are combined to form a heterocycle, wherein said heterocycle is optionally substituted with one or more Rd;
Rc is aryl, optionally substituted with one or more Rd or Re;
Rd is hydrogen, C1-C6 alkyl, oxo, C3-C8 cycloalkyl, or —ORe,
or two Rd when attached to the same carbon atom can form a C3-C12 spirocycle or a 3- to 12-membered spiroheterocycle;
Re is aryl; and
A is hydrogen;
with the proviso that:
when Ra is H and Rb is —C(O)Rc, then Rc cannot be phenyl, 1-naphthyl, 2-naphthyl, or 4-biphenyl.
|