| CPC B01J 31/2208 (2013.01) [B01J 31/2273 (2013.01); B01J 31/2278 (2013.01); C07C 5/31 (2013.01); C07C 43/275 (2013.01); C07C 43/29 (2013.01); C07C 217/90 (2013.01); C07F 15/0046 (2013.01); B01J 2231/324 (2013.01); B01J 2231/543 (2013.01); B01J 2531/821 (2013.01); C07C 2531/22 (2013.01)] | 8 Claims |
|
1. An olefin metathesis reaction which comprises utilizing a ruthenium-based catalyst of formula (II)
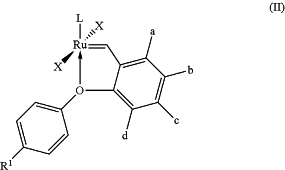 wherein
wherein L is a N-heterocyclic carbene (NHC) ligand or
wherein L is a phosphine ligand selected from the group consisting of tri-isopropylphosphine, tricyclohexylphosphine (PCy3), tricyclopentylphosphine, and phospha-bicycloalkane compounds selected from the group consisting of 9-cyclohexyl-9-phospha-bicyclo-[3.3.1]-nonane (“cyclohexylphobane”), 9-(2,2,4-trimethylpentyl)-9-phospha-bicyclo-[3.3.1]-nonane (“2,2 4-trimethylpentyl phobane”) and 9-isobutyl-9-phospha-bicyclo-[3.3.1]-nonane (“isobutylphobane”),
a, b, c and d are, independently from each other, selected from hydrogen, straight chain or branched C1-C10-alkyl, C1-C10-alkoxy, C1-C10-alkylthio, C1-C10-silyloxy, C1-C10-alkylamino, optionally substituted C6-C14-aryl, optionally substituted C6-C14-aryloxy, or optionally substituted C6-C14-heteroaryl;
R1 is a straight chain or branched alkyl group including C1-C10-alkoxy, C1-C10-alkylthio, C1-C10-silyloxy, C1-C10-alkylamino, C6-C14-aryl, C6-C14-aryloxy, C6-C14-heterocyclic or electron-withdrawing groups (EWG);
X is an anionic ligand independently selected from the group of halogen anions (Cl−, Br−, I−), tetrafluoroborate (BF4−) or acetate (CH3COO−),
wherein the electron-withdrawing groups are halogen atoms, trifluoromethyl (—CF3), nitro (—NO2), sulfinyl (—SO—), sulfonyl (—SO2—), formyl (—CHO), C1-C10-carbonyl, C1-C10-carboxyl, C1-C10-alkylamido, C1-C10-aminocarbonyl, nitrile (—CN) or C1-C10-sulfonamide.
|